The hepatitis E virus ORF3 protein modulates epidermal growth factor receptor trafficking, STAT3 translocation, and the acute-phase response
- PMID: 18448545
- PMCID: PMC2446974
- DOI: 10.1128/JVI.00403-08
The hepatitis E virus ORF3 protein modulates epidermal growth factor receptor trafficking, STAT3 translocation, and the acute-phase response
Abstract
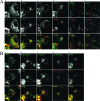
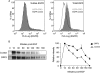
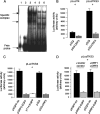
The hepatitis E virus (HEV) causes acute viral hepatitis, but its characterization is hampered by the lack of an efficient in vitro infection system that can be used to study the effects of HEV proteins on cellular processes. Previous studies suggest that the viral ORF3 protein (pORF3) is essential for infection in vivo and is likely to modulate the host response. Here, we report that pORF3 localizes to early and recycling endosomes and causes a delay in the postinternalization trafficking of epidermal growth factor receptor (EGFR) to late endosomes/lysosomes. The cytoplasmic phosphorylated signal transducer and activator of transcription 3 (pSTAT3) proteins require growth factor receptor endocytosis for their translocation from the cytoplasm to nucleus. Consequently, lower levels of pSTAT3 were found in the nuclei of ORF3-expressing Huh7 human hepatoma cells stimulated with EGF. This results in downregulation of the acute-phase response, a major determinant of inflammation in the host. We propose that through its effects on EGFR trafficking, pORF3 prolongs endomembrane growth factor signaling and promotes cell survival. The effects on STAT3 translocation would result in a reduced inflammatory response. Both of these events are likely to contribute positively to viral replication.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

