Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport
- PMID: 18448546
- PMCID: PMC2447115
- DOI: 10.1128/JVI.00557-08
Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport
Abstract
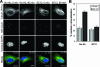
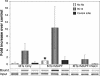
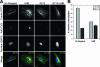
Neutralizing antibodies are commonly elicited by viral infection. Most antibodies that have been characterized block early stages of virus entry that occur before membrane penetration, whereas inhibition of late stages in entry that occurs after membrane penetration has been poorly characterized. Here we provide evidence that the neutralizing antihexon monoclonal antibody 9C12 inhibits adenovirus infection by blocking microtubule-dependent translocation of the virus to the microtubule-organizing center following endosome penetration. These studies identify a previously undescribed mechanism by which neutralizing antibodies block virus infection, a situation that may be relevant for other nonenveloped viruses that use microtubule-dependent transport during cell entry.
Figures
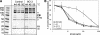

References
-
- Boudin, M. L., and P. Boulanger. 1981. Antibody-triggered dissociation of adenovirus penton capsomer. Virology 113781-786. - PubMed
-
- Burton, D. R. 1995. Phage display. Immunotechnology 187-94. - PubMed
-
- Carey, B., M. K. Staudt, D. Bonaminio, J. C. van der Loo, and B. C. Trapnell. 2007. PU.1 redirects adenovirus to lysosomes in alveolar macrophages, uncoupling internalization from infection. J. Immunol. 1782440-2447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

