Vitamin D receptor activators can protect against vascular calcification
- PMID: 18448587
- PMCID: PMC2488263
- DOI: 10.1681/ASN.2007080902
Vitamin D receptor activators can protect against vascular calcification
Abstract
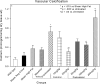
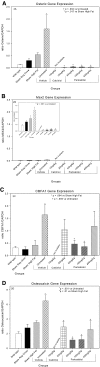
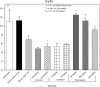
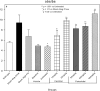
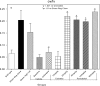
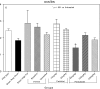
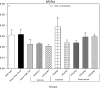
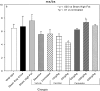
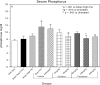
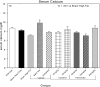
An apparent conflict exists between observational studies that suggest that vitamin D receptor (VDR) activators provide a survival advantage for patients with ESRD and other studies that suggest that they cause vascular calcification. In an effort to explain this discrepancy, we studied the effects of the VDR activators calcitriol and paricalcitol on aortic calcification in a mouse model of chronic kidney disease (CKD)-stimulated atherosclerotic cardiovascular mineralization. At dosages sufficient to correct secondary hyperparathyroidism, calcitriol and paricalcitol were protective against aortic calcification, but higher dosages stimulated aortic calcification. At protective dosages, the VDR activators reduced osteoblastic gene expression in the aorta, which is normally increased in CKD, perhaps explaining this inhibition of aortic calcification. Interpreting the results obtained using this model, however, is complicated by the adynamic bone disorder; both calcitriol and paricalcitol stimulated osteoblast surfaces and rates of bone formation. Therefore, the skeletal actions of the VDR activators may have contributed to their protection against aortic calcification. We conclude that low, clinically relevant dosages of calcitriol and paricalcitol may protect against CKD-stimulated vascular calcification.
Figures
Similar articles
-
Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet.Kidney Int. 2012 Dec;82(12):1261-70. doi: 10.1038/ki.2012.322. Epub 2012 Aug 29. Kidney Int. 2012. PMID: 22932118 Free PMC article.
-
Differential effects of vitamin D receptor activators on vascular calcification in uremic rats.Kidney Int. 2007 Sep;72(6):709-15. doi: 10.1038/sj.ki.5002406. Epub 2007 Jun 27. Kidney Int. 2007. PMID: 17597697
-
Effect of paricalcitol and calcitriol on aortic wall remodeling in uninephrectomized ApoE knockout mice.Am J Physiol Renal Physiol. 2011 Mar;300(3):F772-82. doi: 10.1152/ajprenal.00042.2010. Epub 2010 Dec 15. Am J Physiol Renal Physiol. 2011. PMID: 21159735
-
Vascular calcification in chronic kidney failure: role of vitamin D receptor.Curr Opin Investig Drugs. 2007 Mar;8(3):237-47. Curr Opin Investig Drugs. 2007. PMID: 17408120 Review.
-
Emerging role for the vitamin D receptor activator (VDRA), paricalcitol, in the treatment of secondary hyperparathyroidism.Expert Opin Pharmacother. 2008 Apr;9(6):947-54. doi: 10.1517/14656566.9.6.947. Expert Opin Pharmacother. 2008. PMID: 18377338 Review.
Cited by
-
Epimeric vitamin D and cardiovascular structure and function in advanced CKD and after kidney transplantation.Nephrol Dial Transplant. 2024 Jan 31;39(2):264-276. doi: 10.1093/ndt/gfad168. Nephrol Dial Transplant. 2024. PMID: 37468453 Free PMC article.
-
Mechanisms and treatment of extraosseous calcification in chronic kidney disease.Nat Rev Nephrol. 2011 Jul 19;7(9):509-16. doi: 10.1038/nrneph.2011.91. Nat Rev Nephrol. 2011. PMID: 21769106 Review.
-
Risk factors for radial artery calcification in patients with and without uremia.BMC Nephrol. 2025 Jan 11;26(1):18. doi: 10.1186/s12882-024-03940-0. BMC Nephrol. 2025. PMID: 39799338 Free PMC article.
-
25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification.J Am Soc Nephrol. 2009 Aug;20(8):1805-12. doi: 10.1681/ASN.2008111157. Epub 2009 May 14. J Am Soc Nephrol. 2009. PMID: 19443637 Free PMC article.
-
Supplementation by vitamin D compounds does not affect colonic tumor development in vitamin D sufficient murine models.Arch Biochem Biophys. 2011 Nov;515(1-2):64-71. doi: 10.1016/j.abb.2011.08.011. Epub 2011 Sep 1. Arch Biochem Biophys. 2011. PMID: 21907701 Free PMC article.
References
-
- Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 - PubMed
-
- Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 70: 1858–1865, 2006 - PubMed
-
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 - PubMed
-
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 - PubMed
-
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO4, Ca x PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

