An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs
- PMID: 18448610
- PMCID: PMC2494817
- DOI: 10.1152/ajpregu.00182.2008
An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs
Abstract
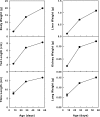
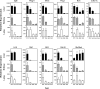
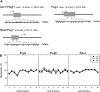
In mammals, somatic growth is rapid in early postnatal life but decelerates with age and eventually halts, thus determining the adult body size of the species. This growth deceleration, which reflects declining proliferation, occurs simultaneously in multiple organs yet appears not to be coordinated by a systemic mechanism. We, therefore, hypothesized that growth deceleration results from a growth-limiting genetic program that is common to multiple tissues. Here, we identified a set of 11 imprinted genes that show down-regulation of mRNA expression with age in multiple organs. For these genes, Igf2, H19, Plagl1, Mest, Peg3, Dlk1, Gtl2, Grb10, Ndn, Cdkn1c, and SLC38a4, the declines show a temporal pattern similar to the decline in growth rate. All 11 genes have been implicated in the control of cell proliferation or somatic growth. Thus, our findings suggest that the declining expression of these genes contributes to coordinate growth deceleration in multiple tissues. We next hypothesized that the coordinate decline in expression of these imprinted genes is caused by altered methylation and consequent silencing of the expressed allele. Contrary to this hypothesis, the methylation status of the promoter regions of Mest, Peg3, and Plagl1 did not change with age. Our findings suggest that a set of growth-regulating imprinted genes is expressed at high levels in multiple tissues in early postnatal life, contributing to rapid somatic growth, but that these genes are subsequently downregulated in multiple tissues simultaneously, contributing to coordinate growth deceleration and cessation, thus imposing a fundamental limit on adult body size.
Figures

Similar articles
-
Temporal and spatial expression of a growth-regulated network of imprinted genes in growth plate.Pediatr Nephrol. 2010 Apr;25(4):617-23. doi: 10.1007/s00467-009-1339-y. Epub 2009 Nov 10. Pediatr Nephrol. 2010. PMID: 19902269
-
An extensive genetic program occurring during postnatal growth in multiple tissues.Endocrinology. 2009 Apr;150(4):1791-800. doi: 10.1210/en.2008-0868. Epub 2008 Nov 26. Endocrinology. 2009. PMID: 19036884 Free PMC article.
-
Evidence That Up-Regulation of MicroRNA-29 Contributes to Postnatal Body Growth Deceleration.Mol Endocrinol. 2015 Jun;29(6):921-32. doi: 10.1210/me.2015-1047. Epub 2015 Apr 13. Mol Endocrinol. 2015. PMID: 25866874 Free PMC article.
-
A set of imprinted genes required for normal body growth also promotes growth of rhabdomyosarcoma cells.Pediatr Res. 2012 Jan;71(1):32-8. doi: 10.1038/pr.2011.6. Pediatr Res. 2012. PMID: 22289848 Free PMC article.
-
Mechanisms limiting body growth in mammals.Endocr Rev. 2011 Jun;32(3):422-40. doi: 10.1210/er.2011-0001. Epub 2011 Mar 25. Endocr Rev. 2011. PMID: 21441345 Free PMC article. Review.
Cited by
-
MicroRNAs and Fetal Brain Development: Implications for Ethanol Teratology during the Second Trimester Period of Neurogenesis.Front Genet. 2012 May 16;3:77. doi: 10.3389/fgene.2012.00077. eCollection 2012. Front Genet. 2012. PMID: 22623924 Free PMC article.
-
Temporal and spatial expression of a growth-regulated network of imprinted genes in growth plate.Pediatr Nephrol. 2010 Apr;25(4):617-23. doi: 10.1007/s00467-009-1339-y. Epub 2009 Nov 10. Pediatr Nephrol. 2010. PMID: 19902269
-
Analysis of neonatal brain lacking ATRX or MeCP2 reveals changes in nucleosome density, CTCF binding and chromatin looping.Nucleic Acids Res. 2014 Jul;42(13):8356-68. doi: 10.1093/nar/gku564. Epub 2014 Jul 2. Nucleic Acids Res. 2014. PMID: 24990380 Free PMC article.
-
Imprinted genes and the environment: links to the toxic metals arsenic, cadmium, lead and mercury.Genes (Basel). 2014 Jun 11;5(2):477-96. doi: 10.3390/genes5020477. Genes (Basel). 2014. PMID: 24921406 Free PMC article.
-
Early life antibiotic exposure affects pancreatic islet development and metabolic regulation.Sci Rep. 2017 Feb 2;7:41778. doi: 10.1038/srep41778. Sci Rep. 2017. PMID: 28150721 Free PMC article.
References
-
- Ahmadian A, Ehn M, Hober S. Pyrosequencing: history, biochemistry and future. Clin Chim Acta 363: 83–94, 2006. - PubMed
-
- Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res 58: 5489–5494, 1998. - PubMed
-
- Bhuiyan ZA, Yatsuki H, Sasaguri T, Joh K, Soejima H, Zhu X, Hatada I, Morisaki H, Morisaki T, Mukai T. Functional analysis of the p57KIP2 gene mutation in Beckwith-Wiedemann syndrome. Hum Genet 104: 205–210, 1999. - PubMed
-
- Chailler P, Briere N. Integration of proliferation and differentiation phenomena during rodent kidney ontogeny. Growth Dev Aging 55: 11–18, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

