The neurogenesis-controlling factor, Pax6, inhibits proliferation and promotes maturation in murine astrocytes
- PMID: 18448636
- PMCID: PMC6670447
- DOI: 10.1523/JNEUROSCI.5074-07.2008
The neurogenesis-controlling factor, Pax6, inhibits proliferation and promotes maturation in murine astrocytes
Abstract
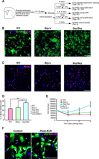
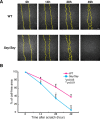
Astrocytes serve various important functions in the CNS, but the molecular mechanisms of their generation and maturation are still enigmatic. Here, we show that Pax6, a key transcription factor that controls neurogenesis, also regulates proliferation, differentiation, and migration of astrocytes in the CNS. We first reveal that Pax6 is expressed in astrocytes during development as well as postnatally in the wild-type mouse. Astrocytes derived from Pax6 homozygous mutants (Sey/Sey) mice exhibited aberrant proliferation together with immature differentiation, both in vivo and in vitro, with higher migration potential in scratch-wound assays in vitro. Furthermore, a larger population of Sey/Sey astrocytes expresses neural stem cell markers such as nestin, Sox2, and prominin-1. These phenotypes of Pax6-deficient astrocytes putatively occur via higher Akt activity. Thus, the breakdown of Pax6 function induces the retention of neural stem-like characteristics and inhibits astrocyte maturation.
Figures





Similar articles
-
Sox2 and Pax6 maintain the proliferative and developmental potential of gliogenic neural stem cells In vitro.Glia. 2011 Nov;59(11):1588-99. doi: 10.1002/glia.21201. Epub 2011 Jul 15. Glia. 2011. PMID: 21766338
-
Forced expression of the motor neuron determinant HB9 in neural stem cells affects neurogenesis.Exp Neurol. 2006 Mar;198(1):167-82. doi: 10.1016/j.expneurol.2005.11.026. Epub 2006 Jan 24. Exp Neurol. 2006. PMID: 16434037
-
The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord.Dev Biol. 2007 May 15;305(2):659-73. doi: 10.1016/j.ydbio.2007.02.012. Epub 2007 Feb 16. Dev Biol. 2007. PMID: 17399698
-
Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator.Stem Cells. 2008 Jul;26(7):1663-72. doi: 10.1634/stemcells.2007-0884. Epub 2008 May 8. Stem Cells. 2008. PMID: 18467663 Review.
-
Role of Pax6 in forebrain regionalization.Brain Res Bull. 2005 Sep 15;66(4-6):387-93. doi: 10.1016/j.brainresbull.2005.02.006. Epub 2005 Feb 24. Brain Res Bull. 2005. PMID: 16144620 Review.
Cited by
-
Horizontal Basal Cell-Specific Deletion of Pax6 Impedes Recovery of the Olfactory Neuroepithelium Following Severe Injury.Stem Cells Dev. 2015 Aug 15;24(16):1923-33. doi: 10.1089/scd.2015.0011. Epub 2015 Apr 28. Stem Cells Dev. 2015. PMID: 25808240 Free PMC article.
-
Regional Immunoreactivity of Pax6 in the Neurogenic Zone After Chronic Prenatal Hypoxia.In Vivo. 2017 Nov-Dec;31(6):1125-1129. doi: 10.21873/invivo.11178. In Vivo. 2017. PMID: 29102934 Free PMC article.
-
Multiomic single-cell profiling identifies critical regulators of postnatal brain.Nat Genet. 2025 Mar;57(3):591-603. doi: 10.1038/s41588-025-02083-8. Epub 2025 Feb 17. Nat Genet. 2025. PMID: 39962241 Free PMC article.
-
Thirty Years' History since the Discovery of Pax6: From Central Nervous System Development to Neurodevelopmental Disorders.Int J Mol Sci. 2022 May 30;23(11):6115. doi: 10.3390/ijms23116115. Int J Mol Sci. 2022. PMID: 35682795 Free PMC article. Review.
-
Aberrant neuronal differentiation and inhibition of dendrite outgrowth resulting from endoplasmic reticulum stress.J Neurosci Res. 2014 Sep;92(9):1122-33. doi: 10.1002/jnr.23389. Epub 2014 Apr 10. J Neurosci Res. 2014. PMID: 24723324 Free PMC article.
References
-
- Abe K, Saito H. Developmental changes in cyclic AMP-stimulated stellation of cultured rat cortical astrocytes. Jpn J Pharmacol. 1997;75:433–438. - PubMed
-
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. - PubMed
-
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751–758. - PubMed
-
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
