Silent synapses in developing rat nucleus tractus solitarii have AMPA receptors
- PMID: 18448639
- PMCID: PMC6670440
- DOI: 10.1523/JNEUROSCI.5355-07.2008
Silent synapses in developing rat nucleus tractus solitarii have AMPA receptors
Abstract
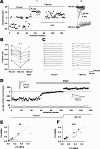
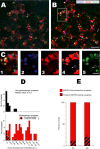
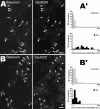
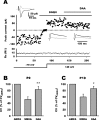
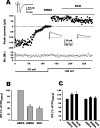
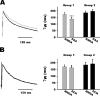
NMDA-only synapses, called silent synapses, are thought to be the initial step in synapse formation in several systems. However, the underlying mechanism and the role in circuit construction are still a matter of dispute. Using combined morphological and electrophysiological approaches, we searched for silent synapses at the level of the nucleus tractus solitarii (NTS), a brainstem structure that is a gateway for many visceral sensory afferent fibers. Silent synapses were detected at birth by using electrophysiological recordings and minimal stimulation protocols. However, anatomical experiments indicated that nearly all, if not all, NTS synapses had AMPA receptors. Based on EPSC fluctuation measurements and differential blockade by low-affinity competitive and noncompetitive glutamate antagonists, we then demonstrated that NTS silent synapses were better explained by glutamate spillover from neighboring fibers and/or slow dynamic of fusion pore opening. Glutamate spillover at immature NTS synapses may favor crosstalk between active synapses during development when glutamate transporters are weakly expressed and contribute to synaptic processing as well as autonomic circuit formation.
Figures
References
-
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. - PubMed
-
- Andresen MC, Kunze DL. Nucleus tractus solitarius-gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
-
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. - PubMed
-
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
