Connexin 43 hemichannels are permeable to ATP
- PMID: 18448647
- PMCID: PMC3638995
- DOI: 10.1523/JNEUROSCI.5048-07.2008
Connexin 43 hemichannels are permeable to ATP
Abstract
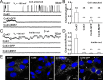
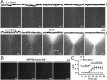
Astrocytes are electrically nonexcitable cells that communicate by means of Ca(2+) signaling. Long-distance intercellular Ca(2+) waves are initiated by release of ATP and activation of purinergic receptors on nearby cells. Previous studies have implicated connexin 43 (Cx43) in ATP release, but definitive proof that ATP exits through Cx43 hemichannels does not exist. Here, through several alternative approaches, we show that ATP anions can permeate through Cx43 hemichannels. First, openings of Cx43 hemichannels were detected in both cell-attached and inside-out patch recordings in C6 cells expressing Cx43, but not in C6 cells expressing Cx43-eGFP (enhanced green fluorescent protein) or a C-terminus truncation mutant of Cx43. Second, Cx43 hemichannel openings were inhibited by three structurally different gap-junction channel blockers, but not by the P2X(7) blocker Brilliant blue G. Third, bioluminescence imaging of ATP combined with single-channel recording in the inside-out patch configuration showed that ATP efflux coincided with channel openings and was absent when the Cx43 hemichannel was closed. Fourth, ion replacement experiments confirmed that Cx43 hemichannels are permeable to ATP. In summary, these observations provide the first direct evidence for efflux of ATP through Cx43 hemichannels. Furthermore, a putative Cx43 hemichannel with characteristics identical to the Cx43 hemichannel in C6 cells was identified in the membrane of hippocampal astrocytes in acutely prepared slices.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
