Zic deficiency in the cortical marginal zone and meninges results in cortical lamination defects resembling those in type II lissencephaly
- PMID: 18448648
- PMCID: PMC6670431
- DOI: 10.1523/JNEUROSCI.5735-07.2008
Zic deficiency in the cortical marginal zone and meninges results in cortical lamination defects resembling those in type II lissencephaly
Abstract
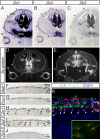
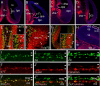
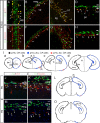
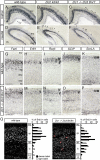
The formation of the highly organized cortical structure depends on the production and correct placement of the appropriate number and types of neurons. The Zic family of zinc-finger transcription factors plays essential roles in regulating the proliferation and differentiation of neuronal progenitors in the medial forebrain and the cerebellum. Examination of the expression of Zic genes demonstrated that Zic1, Zic2, and Zic3 were expressed by the progenitor cells in the septum and cortical hem, the sites of generation of the Cajal-Retzius (CR) cells. Immunohistochemical studies have revealed that Zic proteins were abundantly expressed in the meningeal cells and that the majority of the CR cells distributed in the medial and dorsal cortex also expressed Zic proteins in the mid-late embryonic and postnatal cortical marginal zones. During embryonic cortical development, Zic1/Zic3 double-mutant and hypomorphic Zic2 mutant mice showed a reduction in the number of CR cells in the rostral cortex, whereas the cell number remained unaffected in the caudal cortex. These mutants also showed mislocalization of the CR cells and cortical lamination defects, resembling the changes noted in type II (cobblestone) lissencephaly, throughout the brain. In the Zic1/3 mutant, reduced proliferation of the meningeal cells was observed before the thinner and disrupted organization of the pial basement membrane (BM) with reduced expression of the BM components and the meningeal cell-derived secretory factor. These defects correlated with the changes in the end feet morphology of the radial glial cells. These findings indicate that the Zic genes play critical roles in cortical development through regulating the proliferation of meningeal cells and the pial BM assembly.
Figures




References
-
- Alcántara S, Pozas E, Ibañez CF, Soriano E. BDNF-modulated spatial organization of Cajal-Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cereb Cortex. 2005;16:487–499. - PubMed
-
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci. 2004;26:205–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
