The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice
- PMID: 18448662
- PMCID: PMC7197898
- DOI: 10.1113/expphysiol.2007.040048
The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice
Abstract
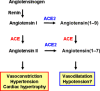
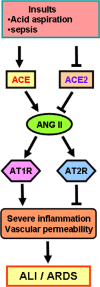
During several months of 2002, severe acute respiratory syndrome (SARS) caused by SARS-coronavirus (SARS-CoV) spread rapidly from China throughout the world, causing more than 800 deaths due to the development of acute respiratory distress syndrome (ARDS), which is the severe form of acute lung injury (ALI). Interestingly, a novel homologue of angiotensin-converting enzyme, termed angiotensin-converting enzyme 2 (ACE2), has been identified as a receptor for SARS-CoV. Angiotensin-converting enzyme and ACE2 share homology in their catalytic domain and provide different key functions in the renin-angiotensin system (RAS). Angiotensin-converting enzyme cleaves angiotensin I to generate angiotensin II, which is a key effector peptide of the system and exerts multiple biological functions, whereas ACE2 reduces angiotensin II levels. Importantly, our recent studies using ACE2 knockout mice have demonstrated that ACE2 protects murine lungs from ARDS. Furthermore, SARS-CoV infections and the Spike protein of the SARS-CoV reduce ACE2 expression. Notably, injection of SARS-CoV Spike into mice worsens acute lung failure in vivo, which can be attenuated by blocking the renin-angiotensin pathway, suggesting that the activation of the pulmonary RAS influences the pathogenesis of ALI/ARDS and SARS.
Figures

Similar articles
-
[Lessons from SARS: a new potential therapy for acute respiratory distress syndrome (ARDS) with angiotensin converting enzyme 2 (ACE2)].Masui. 2008 Mar;57(3):302-10. Masui. 2008. PMID: 18340998 Review. Japanese.
-
Angiotensin-converting enzyme 2 protects from severe acute lung failure.Nature. 2005 Jul 7;436(7047):112-6. doi: 10.1038/nature03712. Nature. 2005. PMID: 16001071 Free PMC article.
-
A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury.Nat Med. 2005 Aug;11(8):875-9. doi: 10.1038/nm1267. Epub 2005 Jul 10. Nat Med. 2005. PMID: 16007097 Free PMC article.
-
Angiotensin-converting enzyme 2 in acute respiratory distress syndrome.Cell Mol Life Sci. 2007 Aug;64(15):2006-12. doi: 10.1007/s00018-007-6228-6. Cell Mol Life Sci. 2007. PMID: 17558469 Free PMC article. Review.
-
Renin-Angiotensin System: An Important Player in the Pathogenesis of Acute Respiratory Distress Syndrome.Int J Mol Sci. 2020 Oct 28;21(21):8038. doi: 10.3390/ijms21218038. Int J Mol Sci. 2020. PMID: 33126657 Free PMC article. Review.
Cited by
-
Role of the Backbenchers of the Renin-Angiotensin System ACE2 and AT2 Receptors in COVID-19: Lessons From SARS.Cureus. 2020 Jun 2;12(6):e8411. doi: 10.7759/cureus.8411. Cureus. 2020. PMID: 32626626 Free PMC article. Review.
-
Smoking and COVID-19: Adding Fuel to the Flame.Int J Mol Sci. 2020 Sep 9;21(18):6581. doi: 10.3390/ijms21186581. Int J Mol Sci. 2020. PMID: 32916821 Free PMC article. Review.
-
Nicotine and the renin-angiotensin system.Am J Physiol Regul Integr Comp Physiol. 2018 Nov 1;315(5):R895-R906. doi: 10.1152/ajpregu.00099.2018. Epub 2018 Aug 8. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 30088946 Free PMC article. Review.
-
Angiotensin converting enzyme-inhibitor reduces colitis severity in an IL-10 knockout model.Dig Dis Sci. 2013 Nov;58(11):3165-77. doi: 10.1007/s10620-013-2825-4. Epub 2013 Aug 15. Dig Dis Sci. 2013. PMID: 23949641 Free PMC article.
-
Will children reveal their secret? The coronavirus dilemma.Eur Respir J. 2020 Jun 25;55(6):2001382. doi: 10.1183/13993003.01382-2020. Print 2020 Jun. Eur Respir J. 2020. PMID: 32398300 Free PMC article.
References
-
- Corvol P, Williams TA & Soubrier F (1995). Peptidyl dipeptidase A: angiotensin I‐converting enzyme. Methods Enzymol 248, 283–305. - PubMed
-
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE et al (2002). Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature 417, 822–828. - PubMed
-
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N et al (2000). A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87, E1–E9. - PubMed
-
- Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI & Lew RA (2004). The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 145, 4703–4711. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

