Detection of pathogenic protozoa in the diagnostic laboratory: result reproducibility, specimen pooling, and competency assessment
- PMID: 18448690
- PMCID: PMC2446938
- DOI: 10.1128/JCM.01666-07
Detection of pathogenic protozoa in the diagnostic laboratory: result reproducibility, specimen pooling, and competency assessment
Abstract
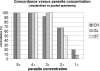
Stool microscopy as performed in clinical parasitology laboratories is a complex procedure with subjective interpretation. Quality assurance (QA) programs often emphasize proficiency testing as an assessment tool. We describe a result reproducibility assessment tool, which can form part of a broader QA program, and which is based on the blinded resubmission of selected clinical samples, using concordance between the reports of the initial and resubmitted specimen as an indicator. Specimens preserved in sodium acetate-acetic acid-formalin can be stored for several months for use in such a program. The presence of multiple protozoa in one specimen does not affect concordance. Some dilution of specimens occurs in this process, and this may explain poor concordance when specimens with low protozoal concentrations are resubmitted. Evaluation of this tool in a large parasitology laboratory revealed concordance rates for pathogenic protozoa (Entamoeba histolytica/Entamoeba dispar, Giardia lamblia, and Dientamoeba fragilis) of about 80%, which may be considered for use as a benchmark value. We also used this tool to demonstrate that when pairs of specimens from one patient are pooled to create a single specimen, concordance between the results of the individual and pooled specimens is high.
Figures

Similar articles
-
Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples.J Clin Microbiol. 2011 Jan;49(1):257-62. doi: 10.1128/JCM.01796-10. Epub 2010 Nov 3. J Clin Microbiol. 2011. PMID: 21048004 Free PMC article.
-
Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories.Clin Microbiol Infect. 2010 Mar;16(3):267-73. doi: 10.1111/j.1469-0691.2009.02782.x. Epub 2009 May 18. Clin Microbiol Infect. 2010. PMID: 19456836
-
Detection of intestinal protozoa in the clinical laboratory.J Clin Microbiol. 2014 Mar;52(3):712-20. doi: 10.1128/JCM.02877-13. Epub 2013 Nov 6. J Clin Microbiol. 2014. PMID: 24197877 Free PMC article. Review.
-
Optimization of routine microscopic and molecular detection of parasitic protozoa in SAF-fixed faecal samples in Sweden.Infect Dis (Lond). 2020 Feb;52(2):87-96. doi: 10.1080/23744235.2019.1682188. Epub 2019 Oct 26. Infect Dis (Lond). 2020. PMID: 31656115
-
Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis.Int J Parasitol. 2007 Jan;37(1):11-20. doi: 10.1016/j.ijpara.2006.09.009. Epub 2006 Oct 12. Int J Parasitol. 2007. PMID: 17070814 Review.
Cited by
-
An audit of inpatient stool ova and parasite (O&P) testing in a multi-hospital health system.J Community Hosp Intern Med Perspect. 2020 Jun 14;10(3):204-209. doi: 10.1080/20009666.2020.1760422. J Community Hosp Intern Med Perspect. 2020. PMID: 32850066 Free PMC article.
-
ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults.Am J Gastroenterol. 2016 May;111(5):602-22. doi: 10.1038/ajg.2016.126. Epub 2016 Apr 12. Am J Gastroenterol. 2016. PMID: 27068718
-
Is there added value from using three serial samples when surveying the occurrence of intestinal parasites in children?Trans R Soc Trop Med Hyg. 2023 Jun 2;117(6):444-450. doi: 10.1093/trstmh/trac132. Trans R Soc Trop Med Hyg. 2023. PMID: 36637010 Free PMC article.
-
Common intestinal parasitic infections among patients living in Riyadh, Saudi Arabia: Prevalence and demographic associations (A cross-sectional retrospective study).Ann Med Surg (Lond). 2022 Apr 28;77:103677. doi: 10.1016/j.amsu.2022.103677. eCollection 2022 May. Ann Med Surg (Lond). 2022. PMID: 35637991 Free PMC article.
-
Dientamoeba fragilis, One of the Neglected Intestinal Protozoa.J Clin Microbiol. 2016 Sep;54(9):2243-50. doi: 10.1128/JCM.00400-16. Epub 2016 Apr 6. J Clin Microbiol. 2016. PMID: 27053676 Free PMC article. Review.
References
-
- Augustin, J. C., and V. Carlier. 2006. Lessons from the organization of a proficiency testing program in food microbiology by interlaboratory comparison: analytical methods in use, impact of methods on bacterial counts and measurement uncertainty of bacterial counts. Food Microbiol. 231-38. - PubMed
-
- Branda, J. A., T. Y. Lin, E. S. Rosenberg, E. F. Halpern, and M. J. Ferraro. 2006. A rational approach to the stool ova and parasite examination. Clin. Infect. Dis. 42972-978. - PubMed
-
- Cox, J. H., G. Ferrari, S. A. Kalams, W. Lopaczynski, N. Oden, and M. P. D'Souza. 2005. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res. Hum. Retrovir. 2168-81. - PubMed
-
- Doern, G. V., A. B. Brueggemann, M. A. Pfaller, and R. N. Jones. 1999. Assessment of laboratory performance with Streptococcus pneumoniae antimicrobial susceptibility testing in the United States: a report from the College of American Pathologists Microbiology Proficiency Survey Program. Arch. Pathol. Lab. Med. 123285-289. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

