Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch
- PMID: 18449562
- PMCID: PMC2904467
- DOI: 10.1007/s00424-008-0501-x
Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch
Abstract
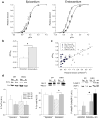
The present study examined the contribution of myofilament contractile proteins to regional function in guinea pig myocardium. We investigated the effect of stretch on myofilament contractile proteins, Ca(2+) sensitivity, and cross-bridge cycling kinetics (K (tr)) of force in single skinned cardiomyocytes isolated from the sub-endocardial (ENDO) or sub-epicardial (EPI) layer. As observed in other species, ENDO cells were stiffer, and Ca(2+) sensitivity of force at long sarcomere length was higher compared with EPI cells. Maximal K (tr) was unchanged by stretch, but was higher in EPI cells possibly due to a higher alpha-MHC content. Submaximal Ca(2+)-activated K (tr) increased only in ENDO cells with stretch. Stretch of skinned ENDO muscle strips induced increased phosphorylation in both myosin-binding protein C and myosin light chain 2. We concluded that transmural MHC isoform expression and differential regulatory protein phosphorylation by stretch contributes to regional differences in stretch modulation of activation in guinea pig left ventricle.
Figures


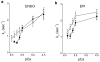
References
-
- Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. - PubMed
-
- Bugaisky LB, Anderson PG, Hall RS, Bishop SP. Differences in myosin isoform expression in the subepicardial and subendocardial myocardium during cardiac hypertrophy in the rat. Circ Res. 1990;66:1127–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

