Flux across [corrected] microneedle-treated skin is increased by increasing charge of naltrexone and naltrexol in vitro
- PMID: 18449628
- PMCID: PMC2757057
- DOI: 10.1007/s11095-008-9578-3
Flux across [corrected] microneedle-treated skin is increased by increasing charge of naltrexone and naltrexol in vitro
Erratum in
- Pharm Res. 2008 Aug;25(8):1964
Abstract
Purpose: The purpose of this investigation was to evaluate the in vitro microneedle (MN) enhanced percutaneous absorption of naltrexone hydrochloride salt (NTX x HCl) compared to naltrexone base (NTX) in hairless guinea pig skin (GP) and human abdominal skin. In a second set of experiments, permeability of the major active metabolite 6-beta-naltrexol base (NTXOL) in the primarily unionized (unprotonated) form at pH 8.5 was compared to the ionized form (pH 4.5).
Methods: In vitro fluxes of NTX, NTX.HCl and ionized and unionized NTXOL were measured through microneedle treated or intact full thickness human and GP skin using a flow through diffusion apparatus. Solubility and diffusion samples were analyzed by HPLC.
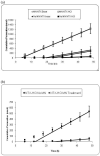
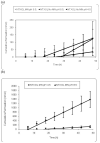
Results: Both GP and human skin show significant increases in flux when treated with 100 MN insertions as compared to intact full thickness skin when treated with NTX.HCl or ionized NTXOL (pH 4.5; p < 0.05). MN increased GP skin permeability for the hydrophilic HCL salt of NTX by tenfold and decreased lag time by tenfold too. Similar results were found using human skin, such that skin permeability to NTX.HCl was elevated to 7.0 x 10(-5) cm/h. Permeability of the primarily unionized (unprotonated) form of NTXOL at pH 8.5 was increased by MN only threefold and lag time was only modestly reduced. However, MN treatment with the primarily ionized (protonated) form of NTXOL at pH 4.5 increased skin permeability fivefold and decreased lag time fourfold.
Conclusion: Enhancement was observed in vitro in both GP and human skin treated with MN compared to intact skin with the salt form of NTX and the ionized form of NTXOL. We conclude that transdermal flux can be optimized by using MN in combination with charged (protonated) drugs that have increased solubility in an aqueous patch reservoir and increased permeability through aqueous pathways created by MN in the skin.
Figures

Similar articles
-
Transdermal delivery of naltrexone and its active metabolite 6-beta-naltrexol in human skin in vitro and guinea pigs in vivo.J Pharm Sci. 2005 Sep;94(9):1965-75. doi: 10.1002/jps.20398. J Pharm Sci. 2005. PMID: 16052561
-
Naltrexone salt selection for enhanced transdermal permeation through microneedle-treated skin.J Pharm Sci. 2012 Aug;101(8):2777-86. doi: 10.1002/jps.23189. Epub 2012 May 24. J Pharm Sci. 2012. PMID: 22628183
-
Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs.J Pharm Sci. 2010 Jul;99(7):3072-80. doi: 10.1002/jps.22083. J Pharm Sci. 2010. PMID: 20166200 Free PMC article.
-
Ex vivo studies for the passive transdermal delivery of low-dose naltrexone from a cream; detection of naltrexone and its active metabolite, 6β-naltrexol, using a novel LC Q-ToF MS assay.Pharm Dev Technol. 2015;20(6):694-701. doi: 10.3109/10837450.2014.915569. Epub 2014 May 2. Pharm Dev Technol. 2015. PMID: 24785567
-
Extended-release intramuscular naltrexone (VIVITROL®): a review of its use in the prevention of relapse to opioid dependence in detoxified patients.CNS Drugs. 2013 Oct;27(10):851-61. doi: 10.1007/s40263-013-0110-x. CNS Drugs. 2013. PMID: 24018540 Review.
Cited by
-
Ex vivo transdermal delivery of 3H-labelled atovaquone solid drug nanoparticles: a comparison of topical, intradermal injection and microneedle assisted administration.Nanoscale Adv. 2023 Oct 17;5(23):6400-6404. doi: 10.1039/d3na00454f. eCollection 2023 Nov 21. Nanoscale Adv. 2023. PMID: 38024306 Free PMC article.
-
Effect of formulation pH on transport of naltrexone species and pore closure in microneedle-enhanced transdermal drug delivery.Mol Pharm. 2013 Jun 3;10(6):2331-9. doi: 10.1021/mp3007083. Epub 2013 May 13. Mol Pharm. 2013. PMID: 23590208 Free PMC article.
-
Thermosensitive Gels Used to Improve Microneedle-Assisted Transdermal Delivery of Naltrexone.Polymers (Basel). 2021 Mar 18;13(6):933. doi: 10.3390/polym13060933. Polymers (Basel). 2021. PMID: 33803552 Free PMC article.
-
Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride.Pharm Res. 2011 Jan;28(1):124-34. doi: 10.1007/s11095-010-0191-x. Epub 2010 Jun 25. Pharm Res. 2011. PMID: 20577787
-
Microneedle-assisted percutaneous delivery of naltrexone hydrochloride in yucatan minipig: in vitro-in vivo correlation.Mol Pharm. 2013 Oct 7;10(10):3745-57. doi: 10.1021/mp400227e. Epub 2013 Sep 23. Mol Pharm. 2013. PMID: 24053426 Free PMC article.
References
-
- Mark TL, Coffey RM, Vandivort-Warren R, Harwood HJ, King EC. U.S. spending for mental health and substance abuse treatment, 1991-2001. Health Aff (Millwood) 2005;(Suppl Web Exclusives):W5-133–W135-142. - PubMed
-
- SAMHSA Results from the 2006 National Survey on Drug Use and Health: National Findings. Department of Health and Human Services: Substance Abuse and Mental Health Services Administration Office of Applied Studies. 2006:1–282.
-
- Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Progress in lipid research. 2003;42:1–36. - PubMed
-
- NIDA Treatment for Drug Abusersin the Criminal Justice System. National Institutes of Health; National Institiute on Drug Abuse. 2006 July;
-
- ACS Cancer Facts and Figures 2007. American Cancer Society. 2007:1–56.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

