Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity
- PMID: 18450774
- PMCID: PMC2538770
- DOI: 10.1113/jphysiol.2008.154187
Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity
Abstract
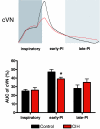
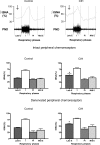
Chronic intermittent hypoxia (CIH) in rats produces changes in the central regulation of cardiovascular and respiratory systems by unknown mechanisms. We hypothesized that CIH (6% O(2) for 40 s, every 9 min, 8 h day(-1)) for 10 days alters the central respiratory modulation of sympathetic activity. After CIH, awake rats (n = 14) exhibited higher levels of mean arterial pressure than controls (101 +/- 3 versus 89 +/- 3 mmHg, n = 15, P < 0.01). Recordings of phrenic, thoracic sympathetic, cervical vagus and abdominal nerves were performed in the in situ working heart-brainstem preparations of control and CIH juvenile rats. The data obtained in CIH rats revealed that: (i) abdominal (Abd) nerves exhibited an additional burst discharge in late expiration; (ii) thoracic sympathetic nerve activity (tSNA) was greater during late expiration than in controls (52 +/- 5 versus 40 +/- 3%; n = 11, P < 0.05; values expressed according to the maximal activity observed during inspiration and the noise level recorded at the end of each experiment), which was not dependent on peripheral chemoreceptors; (iii) the additional late expiratory activity in the Abd nerve correlated with the increased tSNA; (iv) the enhanced late expiratory activity in the Abd nerve unique to CIH rats was accompanied by reduced post-inspiratory activity in cervical vagus nerve compared to controls. The data indicate that CIH rats present an altered pattern of central sympathetic-respiratory coupling, with increased tSNA that correlates with enhanced late expiratory discharge in the Abd nerve. Thus, CIH alters the coupling between the central respiratory generator and sympathetic networks that may contribute to the induced hypertension in this experimental model.
Figures



References
-
- Anrep GV, Pascual W, Rössler R. Respiratory variations of the heart rate I. The reflex mechanism of the respiration arrhythmia. Proc R Soc Lond B Biol Sci. 1936;119:191–217.
-
- Barman SM, Gebber GL. Sympathetic nerve rhythm of brain stem origin. Am J Physiol Regul Integr Comp Physiol. 1980;239:R42–R47. - PubMed
-
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmiters. Physiol Rev. 1995;75:1–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

