Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation
- PMID: 18450953
- PMCID: PMC2488251
- DOI: 10.1210/en.2008-0255
Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation
Abstract
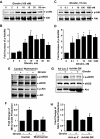
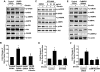
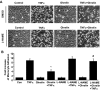
Metabolic syndrome accelerates the atherosclerotic process, and the earliest event of which is endothelial dysfunction. Ghrelin, a newly discovered gastric peptide, improves endothelial function and inhibits proatherogenic changes. In particular, low ghrelin concentration has been associated with several features of metabolic syndrome, including obesity, insulin resistance, and high blood pressure. However, the molecular mechanisms underlying ghrelin vascular actions remain largely unclear. Here, we showed that ghrelin activated endothelial nitric oxide (NO) synthase (eNOS) in cultured endothelial cells (ECs) and in intact vessels. Specifically, ghrelin rapidly induced phosphorylation of eNOS on an activation site and production of NO in human umbilical vein ECs and bovine aortic ECs. The eNOS phosphorylation was also observed in mouse aortas ex vivo perfused with ghrelin and in aortic tissues isolated from mice injected with ghrelin. Mechanistically, ghrelin stimulated AMP-activated protein kinase (AMPK) and Akt activation in cultured ECs and intact vessels. Inhibiting AMPK and Akt with their pharmacological inhibitors, small interference RNA and adenoviruses carried dominant-negative mutants, markedly attenuated ghrelin-induced eNOS activation, and NO production. Furthermore, ghrelin receptor/Gq protein/calcium-dependent pathway mediates activation of AMPK, Akt, and eNOS, and calmodulin-dependent kinase kinase is a potential convergent point to regulate Akt and AMPK activation in ghrelin signaling. Importantly, eNOS activation is critical for ghrelin inhibition of vascular inflammation. Together, both in vitro and in vivo data demonstrate a new role of ghrelin signaling for eNOS activation, and highlight the therapeutic potential for ghrelin to correct endothelial dysfunction associated with atherosclerotic vascular diseases and metabolic syndrome.
Figures



References
-
- Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H 2002 Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation 105:e138–e143 - PubMed
-
- Landmesser U, Hornig B, Drexler H 2004 Endothelial function: a critical determinant in atherosclerosis? Circulation 109(Suppl 1):II27-II33 - PubMed
-
- Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C 2005 Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 111:3489–3493 - PubMed
-
- Zeiher AM 1996 Endothelial vasodilator dysfunction: pathogenetic link to myocardial ischaemia or epiphenomenon? Lancet 348(Suppl 1):s10–s12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

