The heart communicates with the endothelium through the guanylyl cyclase-A receptor: acute handling of intravascular volume in response to volume expansion
- PMID: 18450968
- PMCID: PMC2488219
- DOI: 10.1210/en.2008-0212
The heart communicates with the endothelium through the guanylyl cyclase-A receptor: acute handling of intravascular volume in response to volume expansion
Abstract
Atrial natriuretic peptide (ANP) regulates arterial blood pressure and volume. Its guanylyl cyclase-A (GC-A) receptor is expressed in vascular endothelium and mediates increases in cGMP, but the functional relevance is controversial. Notably, mice with endothelial-restricted GC-A deletion [EC GC-A knockout (KO) mice] exhibit significant chronic hypervolemic hypertension. The present study aimed to characterize the endothelial effects of ANP and their relevance for the acute regulation of intravascular fluid volume. We studied the effect of ANP on microvascular permeability to fluorescein isothiocyanate-labeled albumin (BSA) using intravital microscopy on mouse dorsal skinfold chambers. Local superfusion of ANP (100 nm) increased microvascular fluorescein isothiocyanate-BSA extravasation in control but not EC GC-A KO mice. Intravenous infusion of synthetic ANP (500 ng/kg x min) caused immediate increases in hematocrit in control mice, indicating intravascular volume contraction. In EC GC-A KO mice, the hematocrit responses were not only abolished but even reversed. Furthermore, acute vascular volume expansion, which caused release of endogenous cardiac ANP, did not affect resting central venous pressure of control mice but rapidly and significantly increased central venous pressure of EC GC-A KO mice. In cultured lung endothelial cells, ANP provoked cGMP-dependent protein kinase I-mediated phosphorylation of vasodilator-stimulated phosphoprotein. We conclude that ANP, via GC-A, enhances microvascular endothelial macromolecule permeability in vivo. This effect might be mediated by cGMP-dependent protein kinase I-dependent phosphorylation of vasodilator-stimulated phosphoprotein. Modulation of transcapillary protein and fluid transport may represent one of the most important hypovolemic actions of ANP.
Figures


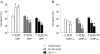
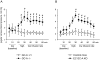
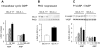
Similar articles
-
Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide.J Clin Invest. 2005 Jun;115(6):1666-74. doi: 10.1172/JCI23360. J Clin Invest. 2005. PMID: 15931395 Free PMC article.
-
Atrial natriuretic peptide enhances microvascular albumin permeability by the caveolae-mediated transcellular pathway.Cardiovasc Res. 2012 Jan 1;93(1):141-51. doi: 10.1093/cvr/cvr279. Epub 2011 Oct 24. Cardiovasc Res. 2012. PMID: 22025581 Free PMC article.
-
Atrial natriuretic peptide-mediated inhibition of microcirculatory endothelial Ca2+ and permeability response to histamine involves cGMP-dependent protein kinase I and TRPC6 channels.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2121-9. doi: 10.1161/ATVBAHA.113.001974. Epub 2013 Jun 27. Arterioscler Thromb Vasc Biol. 2013. PMID: 23814119
-
Endothelial actions of atrial and B-type natriuretic peptides.Br J Pharmacol. 2012 May;166(2):522-31. doi: 10.1111/j.1476-5381.2012.01827.x. Br J Pharmacol. 2012. PMID: 22220582 Free PMC article. Review.
-
Atrial natriuretic factor as a volume regulator.J Clin Pharmacol. 1994 May;34(5):424-6. doi: 10.1002/j.1552-4604.1994.tb04982.x. J Clin Pharmacol. 1994. PMID: 7916352 Review.
Cited by
-
Localization of natriuretic peptide receptors A, B, and C in healthy and diseased mouse kidneys.Pflugers Arch. 2023 Mar;475(3):343-360. doi: 10.1007/s00424-022-02774-9. Epub 2022 Dec 8. Pflugers Arch. 2023. PMID: 36480070 Free PMC article.
-
Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx.Crit Care. 2014 Oct 13;18(5):538. doi: 10.1186/s13054-014-0538-5. Crit Care. 2014. PMID: 25497357 Free PMC article.
-
Atrial natriuretic peptide modulation of albumin clearance and contrast agent permeability in mouse skeletal muscle and skin: role in regulation of plasma volume.J Physiol. 2010 Jan 15;588(Pt 2):325-39. doi: 10.1113/jphysiol.2009.180463. Epub 2009 Nov 30. J Physiol. 2010. PMID: 19948658 Free PMC article.
-
Dorsal skinfold chamber models in mice.GMS Interdiscip Plast Reconstr Surg DGPW. 2017 Jul 10;6:Doc10. doi: 10.3205/iprs000112. eCollection 2017. GMS Interdiscip Plast Reconstr Surg DGPW. 2017. PMID: 28706772 Free PMC article.
-
The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice.J Clin Invest. 2009 Jul;119(7):2019-30. doi: 10.1172/JCI37430. J Clin Invest. 2009. PMID: 19487812 Free PMC article.
References
-
- de Bold AJ, Ma KK, Zhang Y, de Bold ML, Bensimon M, Khoshbaten A 2001 The physiological and pathophysiological modulation of the endocrine function of the heart. Can J Physiol Pharmacol 79:705–714 - PubMed
-
- Kuhn M 2003 Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res 93:700–709 - PubMed
-
- Drewett JG, Garbers DL 1994 The family of guanylyl cyclase receptors and their ligands. Endocr Rev 15:135–162 - PubMed
-
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML 1990 Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70:665–699 - PubMed
-
- Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA 1987 Physiological role of silent receptors of atrial natriuretic factor. Science 238:675–678 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

