The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation
- PMID: 18451107
- PMCID: PMC2335314
- DOI: 10.1101/gad.1642808
The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation
Abstract
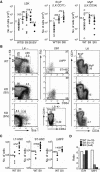
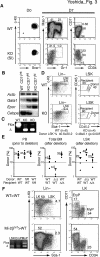
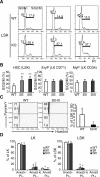
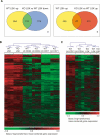
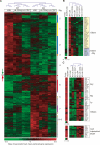
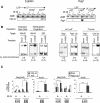
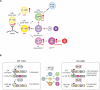
The ability of somatic stem cells to self-renew and differentiate into downstream lineages is dependent on specialized chromatin environments that keep stem cell-specific genes active and key differentiation factors repressed but poised for activation. The epigenetic factors that provide this type of regulation remain ill-defined. Here we provide the first evidence that the SNF2-like ATPase Mi-2beta of the Nucleosome Remodeling Deacetylase (NuRD) complex is required for maintenance of and multilineage differentiation in the early hematopoietic hierarchy. Shortly after conditional inactivation of Mi-2beta, there is an increase in cycling and a decrease in quiescence in an HSC (hematopoietic stem cell)-enriched bone marrow population. These cycling mutant cells readily differentiate into the erythroid lineage but not into the myeloid and lymphoid lineages. Together, these effects result in an initial expansion of mutant HSC and erythroid progenitors that are later depleted as more differentiated proerythroblasts accumulate at hematopoietic sites exhibiting features of erythroid leukemia. Examination of gene expression in the mutant HSC reveals changes in the expression of genes associated with self-renewal and lineage priming and a pivotal role of Mi-2beta in their regulation. Thus, Mi-2beta provides the hematopoietic system with immune cell capabilities as well as with an extensive regenerative capacity.
Figures

References
-
- Adams G.B., Chabner K.T., Alley I.R., Olson D.P., Szczepiorkowski Z.M., Poznansky M.C., Kos C.H., Pollak M.R., Brown E.M., Scadden D.T. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. - PubMed
-
- Adolfsson J., Mansson R., Buza-Vidas N., Hultquist A., Liuba K., Jensen C.T., Bryder D., Yang L., Borge O.J., Thoren L.A., et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Arai F., Hirao A., Ohmura M., Sato H., Matsuoka S., Takubo K., Ito K., Koh G.Y., Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
