Cell cycle control of telomere protection and NHEJ revealed by a ts mutation in the DNA-binding domain of TRF2
- PMID: 18451109
- PMCID: PMC2335317
- DOI: 10.1101/gad.1634008
Cell cycle control of telomere protection and NHEJ revealed by a ts mutation in the DNA-binding domain of TRF2
Abstract
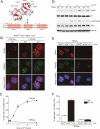
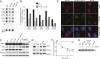
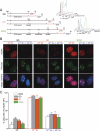
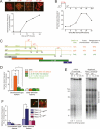
TRF2 is a component of shelterin, the telomere-specific protein complex that prevents DNA damage signaling and inappropriate repair at the natural ends of mammalian chromosomes. We describe a temperature-sensitive (ts) mutation in the Myb/SANT DNA-binding domain of TRF2 that allows controlled and reversible telomere deprotection. At 32 degrees C, TRF2ts was functional and rescued the lethality of TRF2 deletion from conditional TRF2(F/-) mouse embryonic fibroblasts (MEFs). When shifted to the nonpermissive temperature (37 degrees C), TRF2ts cells showed extensive telomere damage resulting in activation of the ATM kinase and nonhomologous end-joining (NHEJ) of chromosome ends. The inactivation of TRF2ts at 37 degrees C was rapid and reversible, permitting induction of short periods (3-6 h) of telomere dysfunction in the G0, G1, and S/G2 phases of the cell cycle. The results indicate that both the induction of telomere dysfunction and the re-establishment of the protected state can take place throughout interphase. In contrast, the processing of dysfunctional telomeres by NHEJ occurred primarily in G1, being repressed in S/G2 in a cyclin-dependent kinase (CDK)-dependent manner.
Figures
References
-
- Aylon Y., Kupiec M. Cell cycle-dependent regulation of double-strand break repair: A role for the CDK. Cell Cycle. 2005;4:259–261. - PubMed
-
- Bailey S.M., Cornforth M.N., Kurimasa A., Chen D.J., Goodwin E.H. Strand-specific postreplicative processing of mammalian telomeres. Science. 2001;293:2462–2465. - PubMed
-
- Bilaud T., Brun C., Ancelin K., Koering C.E., Laroche T., Gilson E. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 1997;17:236–239. - PubMed
-
- Broccoli D., Smogorzewska A., Chong L., de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 1997;17:231–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
