Fibronectin fibrillogenesis regulates three-dimensional neovessel formation
- PMID: 18451110
- PMCID: PMC2335318
- DOI: 10.1101/gad.1643308
Fibronectin fibrillogenesis regulates three-dimensional neovessel formation
Abstract
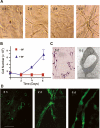
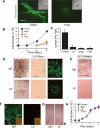
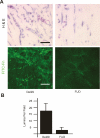
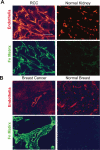
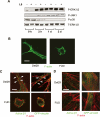
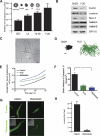
During vasculogenesis and angiogenesis, endothelial cell responses to growth factors are modulated by the compositional and mechanical properties of a surrounding three-dimensional (3D) extracellular matrix (ECM) that is dominated by either cross-linked fibrin or type I collagen. While 3D-embedded endothelial cells establish adhesive interactions with surrounding ligands to optimally respond to soluble or matrix-bound agonists, the manner in which a randomly ordered ECM with diverse physico-mechanical properties is remodeled to support blood vessel formation has remained undefined. Herein, we demonstrate that endothelial cells initiate neovascularization by unfolding soluble fibronectin (Fn) and depositing a pericellular network of fibrils that serve to support cytoskeletal organization, actomyosin-dependent tension, and the viscoelastic properties of the embedded cells in a 3D-specific fashion. These results advance a new model wherein Fn polymerization serves as a structural scaffolding that displays adhesive ligands on a mechanically ideal substratum for promoting neovessel development.
Figures
Similar articles
-
Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis.PLoS One. 2014 Jan 22;9(1):e85178. doi: 10.1371/journal.pone.0085178. eCollection 2014. PLoS One. 2014. PMID: 24465500 Free PMC article.
-
Short-term fibronectin treatment induces endothelial-like and angiogenic properties in monocyte-derived immature dendritic cells: involvement of intracellular VEGF and MAPK regulation.Eur J Cell Biol. 2012 Aug;91(8):640-53. doi: 10.1016/j.ejcb.2012.02.003. Epub 2012 Apr 2. Eur J Cell Biol. 2012. PMID: 22475441
-
pH regulates vascular endothelial growth factor binding to fibronectin: a mechanism for control of extracellular matrix storage and release.J Biol Chem. 2004 Jan 16;279(3):2307-15. doi: 10.1074/jbc.M308482200. Epub 2003 Oct 21. J Biol Chem. 2004. PMID: 14570917
-
Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase.J Biol Chem. 2003 Sep 19;278(38):36661-8. doi: 10.1074/jbc.M301253200. Epub 2003 Jul 3. J Biol Chem. 2003. PMID: 12844492
-
The ins and outs of fibronectin matrix assembly.J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review.
Cited by
-
Role of pericytes in the development of cerebral cavernous malformations.iScience. 2022 Nov 19;25(12):105642. doi: 10.1016/j.isci.2022.105642. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465134 Free PMC article.
-
Collagen I matrix turnover is regulated by fibronectin polymerization.Am J Physiol Cell Physiol. 2010 May;298(5):C1265-75. doi: 10.1152/ajpcell.00341.2009. Epub 2010 Jan 27. Am J Physiol Cell Physiol. 2010. PMID: 20107040 Free PMC article.
-
Tandem mass tag-based serum proteomic profiling revealed diabetic foot ulcer pathogenesis and potential therapeutic targets.Bioengineered. 2022 Feb;13(2):3171-3182. doi: 10.1080/21655979.2022.2027173. Bioengineered. 2022. PMID: 35068329 Free PMC article. Clinical Trial.
-
Differential vascular expression and regulation of oncofetal tenascin-C and fibronectin variants in renal cell carcinoma (RCC): implications for an individualized angiogenesis-related targeted drug delivery.Histochem Cell Biol. 2012 Feb;137(2):195-204. doi: 10.1007/s00418-011-0886-z. Epub 2011 Nov 11. Histochem Cell Biol. 2012. PMID: 22075565
-
MenaINV mediates synergistic cross-talk between signaling pathways driving chemotaxis and haptotaxis.Mol Biol Cell. 2016 Oct 15;27(20):3085-3094. doi: 10.1091/mbc.E16-04-0212. Epub 2016 Aug 24. Mol Biol Cell. 2016. PMID: 27559126 Free PMC article.
References
-
- Ambesi A., Klein R.M., Pumiglia K.M., McKeown-Longo P.J. Anastellin, a fragment of the first type III repeat of fibronectin, inhibits extracellular signal-regulated kinase and causes G(1) arrest in human microvessel endothelial cells. Cancer Res. 2005;65:148–156. - PubMed
-
- Ben-Ze’ev A., Farmer S.R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980;21:365–372. - PubMed
-
- Bershadsky A., Kozlov M., Geiger B. Adhesion-mediated mechanosensitivity: A time to experiment, and a time to theorize. Curr. Opin. Cell Biol. 2006;18:472–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
