PX-RICS mediates ER-to-Golgi transport of the N-cadherin/beta-catenin complex
- PMID: 18451111
- PMCID: PMC2335319
- DOI: 10.1101/gad.1632308
PX-RICS mediates ER-to-Golgi transport of the N-cadherin/beta-catenin complex
Abstract
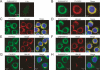
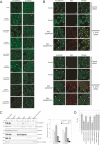
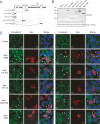
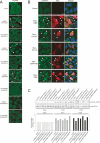
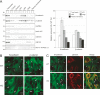
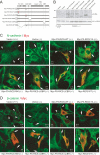
Cadherins mediate Ca2+-dependent cell-cell adhesion. Efficient export of cadherins from the endoplasmic reticulum (ER) is known to require complex formation with beta-catenin. However, the molecular mechanisms underlying this requirement remain elusive. Here we show that PX-RICS, a beta-catenin-interacting GTPase-activating protein (GAP) for Cdc42, mediates ER-to-Golgi transport of the N-cadherin/beta-catenin complex. Knockdown of PX-RICS expression induced the accumulation of the N-cadherin/beta-catenin complex in the ER and ER exit site, resulting in a decrease in cell-cell adhesion. PX-RICS was also required for ER-to-Golgi transport of the fibroblast growth factor-receptor 4 (FGFR4) associated with N-cadherin. PX-RICS-mediated ER-to-Golgi transport was dependent on its interaction with beta-catenin, phosphatidylinositol-4-phosphate (PI4P), Cdc42, and its novel binding partner gamma-aminobutyric acid type A receptor-associated protein (GABARAP). These results suggest that PX-RICS ensures the efficient entry of the N-cadherin/beta-catenin complex into the secretory pathway, and thereby regulates the amount of N-cadherin available for cell adhesion and FGFR4-mediated signaling.
Figures

Similar articles
-
The PX-RICS-14-3-3zeta/theta complex couples N-cadherin-beta-catenin with dynein-dynactin to mediate its export from the endoplasmic reticulum.J Biol Chem. 2010 May 21;285(21):16145-54. doi: 10.1074/jbc.M109.081315. Epub 2010 Mar 22. J Biol Chem. 2010. PMID: 20308060 Free PMC article.
-
PX-RICS, a novel splicing variant of RICS, is a main isoform expressed during neural development.Genes Cells. 2007 Aug;12(8):929-39. doi: 10.1111/j.1365-2443.2007.01101.x. Genes Cells. 2007. PMID: 17663722
-
RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-D-aspartate receptor signaling.J Biol Chem. 2003 Mar 14;278(11):9920-7. doi: 10.1074/jbc.M208872200. Epub 2003 Jan 16. J Biol Chem. 2003. PMID: 12531901
-
Adaptor functions of the Ca2+-binding protein ALG-2 in protein transport from the endoplasmic reticulum.Biosci Biotechnol Biochem. 2019 Jan;83(1):20-32. doi: 10.1080/09168451.2018.1525274. Epub 2018 Sep 27. Biosci Biotechnol Biochem. 2019. PMID: 30259798 Review.
-
Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion.Biochem Biophys Res Commun. 1999 Aug 19;262(1):1-6. doi: 10.1006/bbrc.1999.1122. Biochem Biophys Res Commun. 1999. PMID: 10448058 Review.
Cited by
-
Multiple Functions of ATG8 Family Proteins in Plant Autophagy.Front Cell Dev Biol. 2020 Jun 10;8:466. doi: 10.3389/fcell.2020.00466. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32596242 Free PMC article. Review.
-
Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders.Nat Commun. 2016 Feb 3;7:10594. doi: 10.1038/ncomms10594. Nat Commun. 2016. PMID: 26839058 Free PMC article.
-
Atg8: an autophagy-related ubiquitin-like protein family.Genome Biol. 2011 Jul 27;12(7):226. doi: 10.1186/gb-2011-12-7-226. Genome Biol. 2011. PMID: 21867568 Free PMC article. Review.
-
Lack of GABARAP-Type Proteins Is Accompanied by Altered Golgi Morphology and Surfaceome Composition.Int J Mol Sci. 2020 Dec 23;22(1):85. doi: 10.3390/ijms22010085. Int J Mol Sci. 2020. PMID: 33374830 Free PMC article.
-
LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis.EMBO J. 2010 Jun 2;29(11):1792-802. doi: 10.1038/emboj.2010.74. Epub 2010 Apr 23. EMBO J. 2010. PMID: 20418806 Free PMC article.
References
-
- Barlowe C. Signals for COPII-dependent export from the ER: What’s the ticket out? Trends Cell Biol. 2003;13:295–300. - PubMed
-
- Bruns J.R., Ellis M.A., Jeromin A., Weisz O.A. Multiple roles for phosphatidylinositol 4-kinase in biosynthetic transport in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 2002;277:2012–2018. - PubMed
-
- Cavallaro U., Niedermeyer J., Fuxa M., Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol. 2001;3:650–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
