Pten haploinsufficiency accelerates formation of high-grade astrocytomas
- PMID: 18451155
- PMCID: PMC2760841
- DOI: 10.1158/0008-5472.CAN-07-6867
Pten haploinsufficiency accelerates formation of high-grade astrocytomas
Abstract
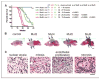
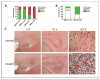
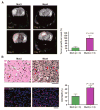
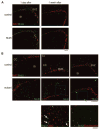
We previously reported that central nervous system (CNS) inactivation of Nf1 and p53 tumor suppressor genes in mice results in the development of low-grade to high-grade progressive astrocytomas. When the tumors achieve high grade, they are frequently accompanied by Akt activation, reminiscent of the frequent association of PTEN mutations in human high-grade glioma. In the present study, we introduced CNS heterozygosity of Pten into the Nf1/p53 astrocytoma model. Resulting mice had accelerated morbidity, shortened survival, and full penetrance of high-grade astrocytomas. Haploinsufficiency of Pten accelerated formation of grade 3 astrocytomas, whereas loss of Pten heterozygosity and Akt activation coincided with progression into grade 4 tumors. These data suggest that successive loss of each Pten allele may contribute to de novo formation of high-grade astrocytoma and progression into glioblastoma, respectively, thus providing insight into the etiology of primary glioblastoma. The presence of ectopically migrating neural stem/progenitor lineage cells in presymptomatic Pten-deficient mutant brains supports the notion that these tumors may arise from stem/progenitor cells.
Figures


References
-
- Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2:616–26. - PubMed
-
- Kleihues P, Cavenee WK, editors. Pathology and genetics of tumours of the nervous system. Lyon: IARC Press; 2000.
-
- Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–33. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Ding H, Roncari L, Shannon P, et al. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 2001;61:3826–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

