The endogenous cannabinoid system modulates nicotine reward and dependence
- PMID: 18451315
- PMCID: PMC2746999
- DOI: 10.1124/jpet.108.138321
The endogenous cannabinoid system modulates nicotine reward and dependence
Abstract
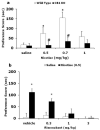
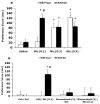
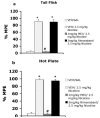
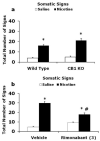
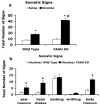
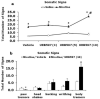
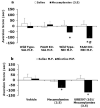
A growing body of evidence suggests that the endogenous cannabinoid system modulates the addictive properties of nicotine, the main component of tobacco that produces rewarding effects. In our study, complementary transgenic and pharmacological approaches were used to test the hypothesis that the endocannabinoid system modulates nicotine reward and dependence. An acute injection of nicotine elicited normal analgesic and hypothermic effects in cannabinoid receptor (CB)(1) knockout (KO) mice and mice treated with the CB(1) antagonist rimonabant. However, disruption of CB(1) receptor signaling blocked nicotine reward, as assessed in the conditioned place preference (CPP) paradigm. In contrast, genetic deletion, or pharmacological inhibition of fatty acid amide hydrolase (FAAH), the enzyme responsible for catabolism of the endocannabinoid anandamide, enhanced the expression of nicotine CPP. Although the expression of spontaneous nicotine withdrawal (14 days, 24 mg/kg/day nicotine) was unaffected in CB(1) KO mice, acute administration of rimonabant (3 mg/kg) ameliorated somatic withdrawal signs in wild-type mice. Increasing endogenous levels of anandamide through genetic or pharmacological approaches exacerbated the physical somatic signs of spontaneous nicotine withdrawal in a milder withdrawal model (7 days, 24 mg/kg/day nicotine). Moreover, FAAH-compromised mice displayed increased conditioned place aversion in a mecamylamine-precipitated model of nicotine withdrawal. These findings indicate that endocannabinoids play a role in the rewarding properties of nicotine as well as nicotine dependence liability. Specifically, increasing endogenous cannabinoid levels magnifies, although disrupting CB(1) receptor signaling, attenuates nicotine reward and withdrawal. Taken together, these results support the hypothesis that cannabinoid receptor antagonists may offer therapeutic advantages to treat tobacco dependence.
Figures
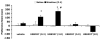

Similar articles
-
The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice.Psychopharmacology (Berl). 2013 Oct;229(4):591-601. doi: 10.1007/s00213-013-3117-6. Epub 2013 May 8. Psychopharmacology (Berl). 2013. PMID: 23652588 Free PMC article.
-
Inhibition of monoacylglycerol lipase reduces nicotine reward in the conditioned place preference test in male mice.Neuropharmacology. 2020 Oct 1;176:108170. doi: 10.1016/j.neuropharm.2020.108170. Epub 2020 May 30. Neuropharmacology. 2020. PMID: 32479813 Free PMC article.
-
Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice.AAPS J. 2009 Jun;11(2):342-52. doi: 10.1208/s12248-009-9110-7. Epub 2009 May 9. AAPS J. 2009. PMID: 19430909 Free PMC article.
-
The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence.Life Sci. 2013 Mar 19;92(8-9):458-62. doi: 10.1016/j.lfs.2012.05.015. Epub 2012 Jun 12. Life Sci. 2013. PMID: 22705310 Free PMC article. Review.
-
An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives.Brain Res Rev. 2007 Jan;53(1):1-16. doi: 10.1016/j.brainresrev.2006.05.003. Epub 2006 Jul 12. Brain Res Rev. 2007. PMID: 16839608 Review.
Cited by
-
Cannabinoid Receptor 1 and Fatty Acid Amide Hydrolase Contribute to Operant Sensation Seeking in Mice.Int J Mol Sci. 2017 Jul 27;18(8):1635. doi: 10.3390/ijms18081635. Int J Mol Sci. 2017. PMID: 28749428 Free PMC article.
-
Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3'-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats.J Pharmacol Exp Ther. 2008 Nov;327(2):482-90. doi: 10.1124/jpet.108.142224. Epub 2008 Aug 25. J Pharmacol Exp Ther. 2008. PMID: 18725543 Free PMC article.
-
Endocannabinoid-mediated synaptic plasticity and addiction-related behavior.Neuropharmacology. 2011 Dec;61(7):1070-87. doi: 10.1016/j.neuropharm.2011.05.034. Epub 2011 Jun 12. Neuropharmacology. 2011. PMID: 21669214 Free PMC article. Review.
-
Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence.Curr Drug Targets. 2013 Jun;14(7):768-76. doi: 10.2174/1389450111314070006. Curr Drug Targets. 2013. PMID: 23614675 Free PMC article. Review.
-
Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction.Pharmacol Ther. 2013 Apr;138(1):84-102. doi: 10.1016/j.pharmthera.2013.01.003. Epub 2013 Jan 16. Pharmacol Ther. 2013. PMID: 23333350 Free PMC article. Review.
References
-
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. - PubMed
-
- Butt C, Alptekin A, Shippenberg T, Oz M. Endogenous cannabinoid anandamide inhibits nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem. 2008;105:1235–1243. - PubMed
-
- Castañé A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav. 2005;81:381–386. - PubMed
-
- Castañé A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

