Atrophin recruits HDAC1/2 and G9a to modify histone H3K9 and to determine cell fates
- PMID: 18451879
- PMCID: PMC2427389
- DOI: 10.1038/embor.2008.67
Atrophin recruits HDAC1/2 and G9a to modify histone H3K9 and to determine cell fates
Abstract
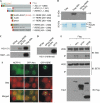
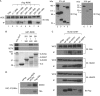
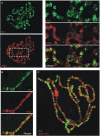
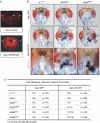
Atrophin family proteins, including the vertebrate arginine-glutamic acid dipeptide repeats protein (RERE) and Drosophila Atrophin (Atro), constitute a new class of nuclear receptor corepressors. Both RERE and Atro share the ELM2 (EGL-27 and MTA1 homology 2) and SANT (SWI3/ADA2/N-CoR/TFIII-B) domains, which are also present in other important transcriptional cofactors. Here, we report that the SANT domain in RERE binds to the histone methyltransferase G9a, and that both the ELM2 and SANT domains orchestrate molecular events that lead to a stable methylation of histone H3-lysine 9. We establish the physiological relevance of these interactions among Atrophin, G9a, and histone deacetylases 1 and 2 in Drosophila by showing that these proteins localize to overlapping chromosomal loci, and act together to suppress wing vein and melanotic-mass formation. This study not only shows a new function of the SANT domain and establishes its connection with the ELM2 domain, but also implies that a similar strategy is used by other ELM2-SANT proteins to repress gene transcription and to exert biological effects.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Histone deacetylase-associating Atrophin proteins are nuclear receptor corepressors.Genes Dev. 2006 Mar 1;20(5):525-30. doi: 10.1101/gad.1393506. Epub 2006 Feb 15. Genes Dev. 2006. PMID: 16481466 Free PMC article.
-
Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1.Mol Cell Biol. 2005 Dec;25(23):10338-51. doi: 10.1128/MCB.25.23.10338-10351.2005. Mol Cell Biol. 2005. PMID: 16287849 Free PMC article.
-
G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis.Genes Dev. 2002 Jul 15;16(14):1779-91. doi: 10.1101/gad.989402. Genes Dev. 2002. PMID: 12130538 Free PMC article.
-
Atrophin proteins: an overview of a new class of nuclear receptor corepressors.Nucl Recept Signal. 2008;6:e009. doi: 10.1621/nrs.06009. Epub 2008 Oct 31. Nucl Recept Signal. 2008. PMID: 19043594 Free PMC article. Review.
-
Steps toward understanding the inheritance of repressive methyl-lysine marks in histones.Cold Spring Harb Symp Quant Biol. 2004;69:171-82. doi: 10.1101/sqb.2004.69.171. Cold Spring Harb Symp Quant Biol. 2004. PMID: 16117647 Review. No abstract available.
Cited by
-
Members of the NODE (Nanog and Oct4-associated deacetylase) complex and SOX-2 promote the initiation of a natural cellular reprogramming event in vivo.Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):6596-601. doi: 10.1073/pnas.1117031109. Epub 2012 Apr 9. Proc Natl Acad Sci U S A. 2012. PMID: 22493276 Free PMC article.
-
Targeting the GFI1/1B-CoREST Complex in Acute Myeloid Leukemia.Front Oncol. 2019 Oct 9;9:1027. doi: 10.3389/fonc.2019.01027. eCollection 2019. Front Oncol. 2019. PMID: 31649884 Free PMC article. Review.
-
MIER1 (mesoderm induction early response 1 homolog (Xenopus laevis)).Atlas Genet Cytogenet Oncol Haematol. 2012 Mar 30;16(2):127-130. doi: 10.4267/2042/46943. Atlas Genet Cytogenet Oncol Haematol. 2012. PMID: 24812577 Free PMC article. No abstract available.
-
Co-expression network of neural-differentiation genes shows specific pattern in schizophrenia.BMC Med Genomics. 2015 May 16;8:23. doi: 10.1186/s12920-015-0098-9. BMC Med Genomics. 2015. PMID: 25981335 Free PMC article.
-
A tale of tailless.Dev Neurosci. 2011;33(1):1-13. doi: 10.1159/000321585. Epub 2010 Dec 2. Dev Neurosci. 2011. PMID: 21124006 Free PMC article. Review.
References
-
- Bowen NJ, Fujita N, Kajita M, Wade PA (2004) Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys Acta 1677: 52–57 - PubMed
-
- Boyer LA, Langer MR, Crowley KA, Tan S, Denu JM, Peterson CL (2002) Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol Cell 10: 935–942 - PubMed
-
- Charroux B, Freeman M, Kerridge S, Baonza A (2006) Atrophin contributes to the negative regulation of epidermal growth factor receptor signaling in Drosophila. Dev Biol 291: 278–290 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

