Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction
- PMID: 18451984
- PMCID: PMC2323106
- DOI: 10.1371/journal.ppat.1000057
Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction
Abstract
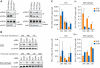
Primate lentiviruses encode four "accessory proteins" including Vif, Vpu, Nef, and Vpr/Vpx. Vif and Vpu counteract the antiviral effects of cellular restrictions to early and late steps in the viral replication cycle. We present evidence that the Vpx proteins of HIV-2/SIV(SM) promote virus infection by antagonizing an antiviral restriction in macrophages. Fusion of macrophages in which Vpx was essential for virus infection, with COS cells in which Vpx was dispensable for virus infection, generated heterokaryons that supported infection by wild-type SIV but not Vpx-deleted SIV. The restriction potently antagonized infection of macrophages by HIV-1, and expression of Vpx in macrophages in trans overcame the restriction to HIV-1 and SIV infection. Vpx was ubiquitylated and both ubiquitylation and the proteasome regulated the activity of Vpx. The ability of Vpx to counteract the restriction to HIV-1 and SIV infection was dependent upon the HIV-1 Vpr interacting protein, damaged DNA binding protein 1 (DDB1), and DDB1 partially substituted for Vpx when fused to Vpr. Our results indicate that macrophage harbor a potent antiviral restriction and that primate lentiviruses have evolved Vpx to counteract this restriction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






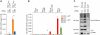
References
-
- Emerman M, Malim MH. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–4. - PubMed
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. - PubMed
-
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;24:406–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

