Central insulin action regulates peripheral glucose and fat metabolism in mice
- PMID: 18451994
- PMCID: PMC2350427
- DOI: 10.1172/JCI31073
Central insulin action regulates peripheral glucose and fat metabolism in mice
Abstract
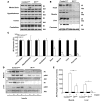
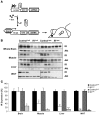
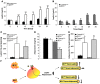
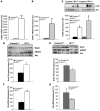
Insulin resistance is a hallmark of type 2 diabetes, and many insights into the functions of insulin have been gained through the study of mice lacking the IR. To gain a better understanding of the role of insulin action in the brain versus peripheral tissues, we created 2 mouse models with inducible IR inactivation, 1 in all tissues including brain (IRDeltawb), and 1 restricted to peripheral tissues (IRDeltaper). While downregulation of IR expression resulted in severe hyperinsulinemia in both models, hyperglycemia was more pronounced in IRDeltawb mice. Both strains displayed a dramatic upregulation of hepatic leptin receptor expression, while only IRDeltaper mice displayed increased hepatic Stat3 phosphorylation and Il6 expression. Despite a similar reduction in IR expression in white adipose tissue (WAT) mass in both models, IRDeltawb mice had a more pronounced reduction in WAT mass and severe hypoleptinemia. Leptin replacement restored hepatic Stat3 phosphorylation and normalized glucose metabolism in these mice, indicating that alterations in glucose metabolism occur largely as a consequence of lipoathrophy upon body-wide IR deletion. Moreover, chronic intracerebroventricular insulin treatment of control mice increased fat mass, fat cell size, and adipose tissue lipoprotein lipase expression, indicating that CNS insulin action promotes lipogenesis. These studies demonstrate that central insulin action plays an important role in regulating WAT mass and glucose metabolism via hepatic Stat3 activation.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

