Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice
- PMID: 18452147
- PMCID: PMC2749317
- DOI: 10.1002/hep.22226
Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice
Abstract
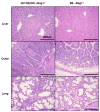
We recently reported that mice with a T cell-restricted expression of a dominant negative form of transforming growth factor beta receptor type II (dnTGFbetaRII) spontaneously develop autoimmune cholangitis that resembles human primary biliary cirrhosis (PBC), including antimitochondrial antibodies (AMAs) and extensive portal CD4(+) and CD8(+) lymphocytic infiltrates. On the basis of these data, we performed a series of experiments to determine whether the pathology was secondary to direct dnTGFbetaRII disruption of the liver and/or alternatively the appearance of autoreactive T cells. First, using dnTGFbetaRIIRag1(-/-) mice, we noted a normal hepatic and biliary structure. Hence, we performed a rigorous series of adoptive transfer studies, transferring Ly5.1(+) unfractionated spleen cell CD4(+) or CD8(+) T cells from dnTGFbetaRII mice into B6/Rag(-/-) (Ly 5.2) recipients. In unmanipulated dnTGFbetaRII mice, there was a marked increase in CD4(+) and CD8(+) T cell biliary infiltrates with AMA. Indeed, B6/Rag(-/-) recipients of dnTGFbetaRII unfractionated cells develop features of liver disease similar to PBC, suggesting that splenic loss of self-tolerance alone is sufficient to cause disease in this model and therefore that there is no specific abnormality in the biliary targets required for appearance of disease. More importantly, adoptive transfer of CD8(+) but not CD4(+) T cells into B6/Rag(-/-) mice led to liver histopathology remarkably similar to PBC, emphasizing a prominent role for CD8 T cell-mediated pathogenesis. In contrast, B6/Rag(-/-) recipients of CD4(+) T cells from dnTGFbetaRII mice predominantly developed inflammatory bowel disease associated with higher levels of serum interferon gamma and tumor necrosis factor alpha.
Conclusion: These data suggest that in this model of PBC, autoreactive CD8(+) cells destroy bile ducts.
Figures




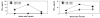


References
-
- Ueno Y, Moritoki Y, Shimosegawa T, Gershwin ME. Primary biliary cirrhosis: what we know and what we want to know about human PBC and spontaneous PBC mouse models. J Gastroenterol. 2007;42:189–195. - PubMed
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. - PubMed
-
- Chuang YH, Lian ZX, Cheng CM, Lan RY, Yang GX, Moritoki Y, Chiang BL, et al. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun. 2005;25:126–132. - PubMed
-
- Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. - PubMed
-
- Van de Water J, Fregeau D, Davis P, Ansari A, Danner D, Leung P, Coppel R, et al. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol. 1988;141:2321–2324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
