PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids
- PMID: 18452513
- PMCID: PMC2615194
- DOI: 10.1111/j.1365-2958.2008.06254.x
PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids
Abstract
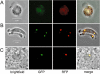
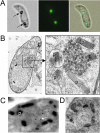
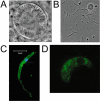
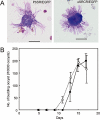
Crystalloids are transient organelles that form in developing malaria ookinetes and disappear after ookinete-to-oocyst transition. Their origins and functions remain poorly understood. The Plasmodium berghei scavenger receptor-like protein PbSR is essential for mosquito-to-host transmission of the parasite: PbSR knockout parasites produce normal numbers of oocysts that fail to form sporozoites, pointing to a role for PbSR in the oocyst during sporogony. Here, using fluorescent protein tagging and targeted gene disruption, we show that PbSR is synthesized in macrogametocytes, gets targeted to the crystalloids of developing ookinetes and is involved in crystalloid formation. While oocyst sporulation rates of PbSR knockout parasites are highly reduced in parasite-infected mosquitoes, sporulation rates in vitro are not adversely affected, supporting the view that mosquito factors could be involved in the PbSR loss-of-function phenotype. These findings are the first to identify a parasite protein involved with the crystalloid organelle, and suggest a novel protein-trafficking mechanism to deliver PbSR to the oocysts.
Figures


Similar articles
-
Malaria crystalloids: specialized structures for parasite transmission?Trends Parasitol. 2011 Mar;27(3):106-10. doi: 10.1016/j.pt.2010.12.004. Epub 2011 Jan 13. Trends Parasitol. 2011. PMID: 21237711 Free PMC article.
-
Plasmodium berghei crystalloids contain multiple LCCL proteins.Mol Biochem Parasitol. 2010 Mar;170(1):49-53. doi: 10.1016/j.molbiopara.2009.11.008. Epub 2009 Nov 22. Mol Biochem Parasitol. 2010. PMID: 19932717 Free PMC article.
-
SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development.Mol Microbiol. 2003 Jul;49(2):319-29. doi: 10.1046/j.1365-2958.2003.03566.x. Mol Microbiol. 2003. PMID: 12828632
-
Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells?Malar J. 2005 Feb 25;4:15. doi: 10.1186/1475-2875-4-15. Malar J. 2005. PMID: 15733320 Free PMC article. Review.
-
Plasmodium Oocysts: Overlooked Targets of Mosquito Immunity.Trends Parasitol. 2016 Dec;32(12):979-990. doi: 10.1016/j.pt.2016.08.012. Epub 2016 Sep 14. Trends Parasitol. 2016. PMID: 27639778 Review.
Cited by
-
Maternally supplied S-acyl-transferase is required for crystalloid organelle formation and transmission of the malaria parasite.Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7183-8. doi: 10.1073/pnas.1522381113. Epub 2016 Jun 14. Proc Natl Acad Sci U S A. 2016. PMID: 27303037 Free PMC article.
-
Biogenesis of the crystalloid organelle in Plasmodium involves microtubule-dependent vesicle transport and assembly.Int J Parasitol. 2015 Jul;45(8):537-47. doi: 10.1016/j.ijpara.2015.03.002. Epub 2015 Apr 18. Int J Parasitol. 2015. PMID: 25900212 Free PMC article.
-
Dynamic protein S-palmitoylation mediates parasite life cycle progression and diverse mechanisms of virulence.Crit Rev Biochem Mol Biol. 2017 Apr;52(2):145-162. doi: 10.1080/10409238.2017.1287161. Epub 2017 Feb 20. Crit Rev Biochem Mol Biol. 2017. PMID: 28228066 Free PMC article. Review.
-
Malaria parasite development in the mosquito and infection of the mammalian host.Annu Rev Microbiol. 2009;63:195-221. doi: 10.1146/annurev.micro.091208.073403. Annu Rev Microbiol. 2009. PMID: 19575563 Free PMC article. Review.
-
Towards genome-wide experimental genetics in the in vivo malaria model parasite Plasmodium berghei.Pathog Glob Health. 2015 Mar;109(2):46-60. doi: 10.1179/2047773215Y.0000000006. Epub 2015 Mar 19. Pathog Glob Health. 2015. PMID: 25789828 Free PMC article. Review.
References
-
- Al-Olayan EM, Beetsma AL, Butcher GA, Sinden RE, Hurd H. Complete development of the mosquito phases of the malaria parasite in vitro. Science. 2002;295:677–679. - PubMed
-
- Arai M, Billker O, Morris HR, Panico M, Delcroix M, Dixon D, et al. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol Biochem Parasitol. 2001;116:17–24. - PubMed
-
- Claudianos C, Dessens JT, Trueman HE, Arai M, Mendoza J, Butcher GA, et al. A malaria scavenger receptor-like protein essential for parasite development. Mol Microbiol. 2002;45:1473–1484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

