YscU cleavage and the assembly of Yersinia type III secretion machine complexes
- PMID: 18452514
- PMCID: PMC3538813
- DOI: 10.1111/j.1365-2958.2008.06247.x
YscU cleavage and the assembly of Yersinia type III secretion machine complexes
Abstract
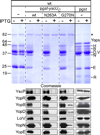
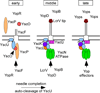
YscU, a component of the Yersinia type III secretion machine, promotes auto-cleavage at asparagine 263 (N263). Mutants with an alanine substitution at yscU codon 263 displayed secretion defects for some substrates (LcrV, YopB and YopD); however, transport of effector proteins into host cells (YopE, YopH, YopM) continued to occur. Two yscU mutations were isolated that, unlike N263A, completely abolished type III secretion; YscU(G127D) promoted auto-cleavage at N263, whereas YscU(G270N) did not. When fused to glutathione S-transferase (Gst), the YscU C-terminal cytoplasmic domain promoted auto-cleavage and Gst-YscU(C) also exerted a dominant-negative phenotype by blocking type III secretion. Gst-YscU(C/N263A) caused a similar blockade and Gst-YscU(C/G270N) reduced secretion. Gst-YscU(C) and Gst-YscU(C/N263A) bound YscL, the regulator of the ATPase YscN, whereas Gst-YscU(C/G270N) did not. When isolated from Yersinia, Gst-YscU(C) and Gst-YscU(C/N263A) associated with YscK-YscL-YscQ; however, Gst-YscU(C/G270N) interacted predominantly with the machine component YscO, but not with YscK-YscL-YscQ. A model is proposed whereby YscU auto-cleavage promotes interaction with YscL and recruitment of ATPase complexes that initiate type III secretion.
Figures







Similar articles
-
YscU/FlhB of Yersinia pseudotuberculosis Harbors a C-terminal Type III Secretion Signal.J Biol Chem. 2015 Oct 23;290(43):26282-91. doi: 10.1074/jbc.M114.633677. Epub 2015 Sep 3. J Biol Chem. 2015. PMID: 26338709 Free PMC article.
-
YscU recognizes translocators as export substrates of the Yersinia injectisome.EMBO J. 2007 Jun 20;26(12):3015-24. doi: 10.1038/sj.emboj.7601731. Epub 2007 May 17. EMBO J. 2007. PMID: 17510628 Free PMC article.
-
Impassable YscP substrates and their impact on the Yersinia enterocolitica type III secretion pathway.J Bacteriol. 2008 Sep;190(18):6204-16. doi: 10.1128/JB.00467-08. Epub 2008 Jul 18. J Bacteriol. 2008. PMID: 18641141 Free PMC article.
-
Regulation of the Yersinia type III secretion system: traffic control.Front Cell Infect Microbiol. 2013 Feb 6;3:4. doi: 10.3389/fcimb.2013.00004. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23390616 Free PMC article. Review.
-
The virulence plasmid of Yersinia, an antihost genome.Microbiol Mol Biol Rev. 1998 Dec;62(4):1315-52. doi: 10.1128/MMBR.62.4.1315-1352.1998. Microbiol Mol Biol Rev. 1998. PMID: 9841674 Free PMC article. Review.
Cited by
-
The Chlamydial Type III Secretion Mechanism: Revealing Cracks in a Tough Nut.Front Microbiol. 2010 Oct 19;1:114. doi: 10.3389/fmicb.2010.00114. eCollection 2010. Front Microbiol. 2010. PMID: 21738522 Free PMC article.
-
A dominant-negative needle mutant blocks type III secretion of early but not late substrates in Yersinia.Mol Microbiol. 2010 Apr;76(1):236-59. doi: 10.1111/j.1365-2958.2010.07096.x. Epub 2010 Feb 28. Mol Microbiol. 2010. PMID: 20199604 Free PMC article.
-
Orf1B controls secretion of T3SS proteins and contributes to Edwardsiella piscicida adhesion to epithelial cells.Vet Res. 2022 Jun 13;53(1):40. doi: 10.1186/s13567-022-01057-6. Vet Res. 2022. PMID: 35692056 Free PMC article.
-
YscU/FlhB of Yersinia pseudotuberculosis Harbors a C-terminal Type III Secretion Signal.J Biol Chem. 2015 Oct 23;290(43):26282-91. doi: 10.1074/jbc.M114.633677. Epub 2015 Sep 3. J Biol Chem. 2015. PMID: 26338709 Free PMC article.
-
Role of autocleavage in the function of a type III secretion specificity switch protein in Salmonella enterica serovar Typhimurium.mBio. 2015 Oct 13;6(5):e01459-15. doi: 10.1128/mBio.01459-15. mBio. 2015. PMID: 26463164 Free PMC article.
References
-
- Agrain C, Sorg I, Paroz C, Cornelis GR. Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol. Microbiol. 2005;57:1415–1427. - PubMed
-
- Allaoui A, Schulte R, Cornelis GR. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol. Microbiol. 1995;18:343–355. - PubMed
-
- Anderson DM, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

