Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins
- PMID: 18452557
- PMCID: PMC11159094
- DOI: 10.1111/j.1349-7006.2008.00840.x
Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins
Abstract
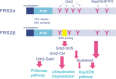
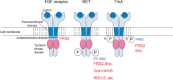
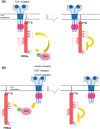
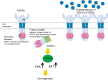
The FRS2 family of adaptor/scaffold proteins has two members, FRS2alpha and FRS2beta. Both proteins contain N-terminal myristylation sites for localization on the plasma membrane and a PTB domain for binding to limited species of receptor tyrosine kinases (RTKs), including the FGF receptor, the neurotophin receptor, RET, and ALK. Activation of these RTKs allows FRS2 proteins to become phosphorylated of tyrosine residues and then bind to Grb2 and Shp2, a SH2 domain-containing adaptor and a tyrosine phosphatase, respectively. Subsequently, Shp2 activates a Ras/ERK pathway and Grb2 activates a Ras/ERK, phosphatidyl inositol (PI)-3 kinase and ubiquitination/degradation pathways by binding to SOS, Gab1, and Cbl via the SH3 domains of Grb2. FRS2alpha acts as 'a conning center' in FGF signaling mainly because it induces sustained levels of activation of ERK via Shp2-binding sites and Grb2-binding sites, though the contribution of the former is greater. Indeed, FRS2alpha knockout mice and mice with mutated Shp2-binding sites exhibit a variety of phenotypes due to defects in FGF signaling in vivo. Although FRS2beta binds to the EGF receptor, it does not induce tyrosine phosphorylation on the receptor. Instead, it inhibits EGF signaling, resulting in inhibition of EGF-induced cell proliferation and cell transformation. Based on these findings, the involvement of FRS2 proteins in tumorigenesis should be studied extensively to be validated as candidate biomarkers for the effectiveness of treatments targeting RTKs such as the FGF receptor and EGF receptor.
Figures


References
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103: 211–25. - PubMed
-
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science 1997; 278: 2075–80. - PubMed
-
- Pawson T. Dynamic control of signaling by modular adaptor proteins. Curr Opin Cell Biol 2007; 19: 112–16. - PubMed
-
- Gu H, Neel BG. The ‘Gab’ in signal transduction. Trends Cell Biol 2003; 13: 122–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

