Induction of leukemia-specific antibodies by immunotherapy with leukemia-cell-derived heat shock protein 70
- PMID: 18452562
- PMCID: PMC11159868
- DOI: 10.1111/j.1349-7006.2008.00829.x
Induction of leukemia-specific antibodies by immunotherapy with leukemia-cell-derived heat shock protein 70
Abstract
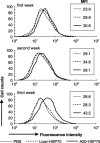
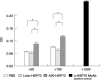
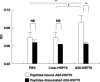
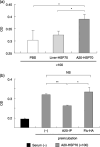
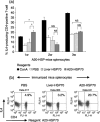
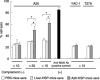
Cancer immunotherapy using heat shock protein (HSP) derived from autologous tumor requires cluster of differentiation (CD)4(+) as well as CD8(+) T-cells for the prolongation of patient survival, suggesting that a humoral immune response through CD4(+) T-cells is important in addition to cellular immunity. However, the role of humoral responses in HSP-based autologous tumor immunotherapy remains unclear. In the present study, we investigated whether leukemia-specific antibodies and antibody-mediated cytotoxicity against autologous leukemia cells have a crucial role in a mouse A20 leukemia model by immunizing A20-derived HSP70. Immunization with A20-derived HSP70 induced the production of anti-A20-antibodies and the antibodies recognized HSP70-binding peptides derived from A20. One of those was a major histocompatibility complex (MHC) class-I binding peptide, which has been clarified as the target peptide of CD8+ cytotoxic T-cells (CTL) against A20. The anti-A20-antibodies produced by immunization with A20-derived HSP70 induced complement-dependent cytotoxicity (CDC) against A20 in vitro. In addition, immunization with A20-derived HSP70 increased intracellular interleukin-4 (IL4)-production of CD4(+) T-cells, confirming the activation of type-2 helper T-cells. Taken together, immunization with leukemia-cell-derived HSP70 induces antibodies against leukemia-cell-specific peptides and might play a crucial role in the eradication of leukemia cells by CDC in mice. These findings will enable future establishment of a novel therapeutic strategy using antileukemia antibodies in HSP-based autologous tumor immunotherapy.
Figures
Similar articles
-
Combined use of dendritic cells enhances specific antileukemia immunity by leukemia cell-derived heat shock protein 70 in a mouse model with minimal residual leukemia cells.Int J Hematol. 2006 Dec;84(5):449-58. doi: 10.1532/IJH97.06003. Int J Hematol. 2006. PMID: 17189229
-
Immunotherapy using heat-shock protein preparations of leukemia cells after syngeneic bone marrow transplantation in mice.Blood. 2001 Sep 15;98(6):1852-7. doi: 10.1182/blood.v98.6.1852. Blood. 2001. PMID: 11535521
-
Vaccination with a DNA vaccine based on human PSCA and HSP70 adjuvant enhances the antigen-specific CD8+ T-cell response and inhibits the PSCA+ tumors growth in mice.J Gene Med. 2007 Aug;9(8):715-26. doi: 10.1002/jgm.1067. J Gene Med. 2007. PMID: 17595048
-
Autologous Hsp70 immunization induces anti-tumor immunity and increases longevity and survival of tumor-bearing mice.Neoplasma. 2009;56(3):259-68. doi: 10.4149/neo_2009_03_259. Neoplasma. 2009. PMID: 19309230
-
The dual-function of HSP70 in immune response and tumor immunity: from molecular regulation to therapeutic innovations.Front Immunol. 2025 Apr 14;16:1587414. doi: 10.3389/fimmu.2025.1587414. eCollection 2025. Front Immunol. 2025. PMID: 40297581 Free PMC article. Review.
Cited by
-
Heat shock protein vaccination and directed IL-2 therapy amplify tumor immunity rapidly following bone marrow transplantation in mice.Blood. 2014 May 8;123(19):3045-55. doi: 10.1182/blood-2013-08-520775. Epub 2014 Mar 31. Blood. 2014. PMID: 24687086 Free PMC article.
-
Early vaccination protects against childhood leukemia: A systematic review and meta-analysis.Sci Rep. 2017 Nov 22;7(1):15986. doi: 10.1038/s41598-017-16067-0. Sci Rep. 2017. PMID: 29167460 Free PMC article.
-
CD91-Dependent Modulation of Immune Responses by Heat Shock Proteins: A Role in Autoimmunity.Autoimmune Dis. 2012;2012:863041. doi: 10.1155/2012/863041. Epub 2012 Nov 19. Autoimmune Dis. 2012. PMID: 23209886 Free PMC article.
-
Targeting Hsp70: A possible therapy for cancer.Cancer Lett. 2016 Apr 28;374(1):156-166. doi: 10.1016/j.canlet.2016.01.056. Epub 2016 Feb 17. Cancer Lett. 2016. PMID: 26898980 Free PMC article. Review.
References
-
- Przepiorka D, Srivastava PK. Heat shock protein‐peptide complexes as immunotherapy for human cancer. Mol Med Today 1998; 4: 478–84. - PubMed
-
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet 2003; 362: 469–76. - PubMed
-
- Srivastava PK, Udono H, Blachere N, Li Z. Heat shock proteins transfer peptide during antigen processing and CTL priming. Immunogenetics 1994; 39: 93–8. - PubMed
-
- Srivastava PK. Roles of heat‐shock proteins in innate and adaptive immunity. Nat Rev Immunol 2002; 2: 185–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

