Identification of a novel alpha(1-->6) mannopyranosyltransferase MptB from Corynebacterium glutamicum by deletion of a conserved gene, NCgl1505, affords a lipomannan- and lipoarabinomannan-deficient mutant
- PMID: 18452585
- PMCID: PMC2440535
- DOI: 10.1111/j.1365-2958.2008.06265.x
Identification of a novel alpha(1-->6) mannopyranosyltransferase MptB from Corynebacterium glutamicum by deletion of a conserved gene, NCgl1505, affords a lipomannan- and lipoarabinomannan-deficient mutant
Abstract
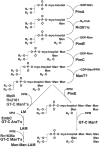
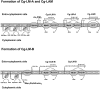
Mycobacterium tuberculosis and Corynebacterium glutamicum share a similar cell wall structure and orthologous enzymes involved in cell wall assembly. Herein, we have studied C. glutamicum NCgl1505, the orthologue of putative glycosyltransferases Rv1459c from M. tuberculosis and MSMEG3120 from Mycobacterium smegmatis. Deletion of NCgl1505 resulted in the absence of lipomannan (Cg-LM-A), lipoarabinomannan (Cg-LAM) and a multi-mannosylated polymer (Cg-LM-B) based on a 1,2-di-O-C(16)/C(18:1)-(alpha-D-glucopyranosyluronic acid)-(1-->3)-glycerol (GlcAGroAc(2)) anchor, while syntheses of triacylated-phosphatidyl-myo-inositol dimannoside (Ac(1)PIM(2)) and Man(1)GlcAGroAc(2) were still abundant in whole cells. Cell-free incubation of C. glutamicum membranes with GDP-[(14)C]Man established that C. glutamicum synthesized a novel alpha(1-->6)-linked linear form of Cg-LM-A and Cg-LM-B from Ac(1)PIM(2) and Man(1)GlcAGroAc(2) respectively. Furthermore, deletion of NCgl1505 also led to the absence of in vitro synthesized linear Cg-LM-A and Cg-LM-B, demonstrating that NCgl1505 was involved in core alpha(1-->6) mannan biosynthesis of Cg-LM-A and Cg-LM-B, extending Ac(1)PI[(14)C]M(2) and [(14)C]Man(1)GlcAGroAc(2) primers respectively. Use of the acceptor alpha-D-Manp-(1-->6)-alpha-D-Manp-O-C(8) in an in vitro cell-free assay confirmed NCgl1505 as an alpha(1-->6) mannopyranosyltransferase, now termed MptB. While Rv1459c and MSMEG3120 demonstrated similar in vitroalpha(1-->6) mannopyranosyltransferase activity, deletion of the Rv1459c homologue in M. smegmatis did not result in loss of mycobacterial LM/LAM, indicating a functional redundancy for this enzyme in mycobacteria.
Figures





Similar articles
-
Inactivation of Corynebacterium glutamicum NCgl0452 and the role of MgtA in the biosynthesis of a novel mannosylated glycolipid involved in lipomannan biosynthesis.J Biol Chem. 2007 Feb 16;282(7):4561-4572. doi: 10.1074/jbc.M608695200. Epub 2006 Dec 19. J Biol Chem. 2007. PMID: 17179146
-
Identification of an alpha(1-->6) mannopyranosyltransferase (MptA), involved in Corynebacterium glutamicum lipomanann biosynthesis, and identification of its orthologue in Mycobacterium tuberculosis.Mol Microbiol. 2007 Sep;65(6):1503-17. doi: 10.1111/j.1365-2958.2007.05884.x. Epub 2007 Aug 21. Mol Microbiol. 2007. PMID: 17714444 Free PMC article.
-
Structural characterization and functional properties of a novel lipomannan variant isolated from a Corynebacterium glutamicum pimB' mutant.Antonie Van Leeuwenhoek. 2008 Aug;94(2):277-87. doi: 10.1007/s10482-008-9243-1. Epub 2008 Apr 18. Antonie Van Leeuwenhoek. 2008. PMID: 18421567 Free PMC article.
-
Molecular basis of phosphatidyl-myo-inositol mannoside biosynthesis and regulation in mycobacteria.J Biol Chem. 2010 Oct 29;285(44):33577-83. doi: 10.1074/jbc.R110.168328. Epub 2010 Aug 27. J Biol Chem. 2010. PMID: 20801880 Free PMC article. Review.
-
Cell wall synthesizing complexes in Mycobacteriales.Curr Opin Microbiol. 2024 Jun;79:102478. doi: 10.1016/j.mib.2024.102478. Epub 2024 Apr 22. Curr Opin Microbiol. 2024. PMID: 38653035 Review.
Cited by
-
The lipoprotein LpqW is essential for the mannosylation of periplasmic glycolipids in Corynebacteria.J Biol Chem. 2012 Dec 14;287(51):42726-38. doi: 10.1074/jbc.M112.373415. Epub 2012 Oct 22. J Biol Chem. 2012. PMID: 23091062 Free PMC article.
-
Characterization of the Corynebacterium glutamicum deltapimB' deltamgtA double deletion mutant and the role of Mycobacterium tuberculosis orthologues Rv2188c and Rv0557 in glycolipid biosynthesis.J Bacteriol. 2009 Jul;191(13):4465-72. doi: 10.1128/JB.01729-08. Epub 2009 Apr 24. J Bacteriol. 2009. PMID: 19395496 Free PMC article.
-
The cell envelope glycoconjugates of Mycobacterium tuberculosis.Crit Rev Biochem Mol Biol. 2014 Sep-Oct;49(5):361-99. doi: 10.3109/10409238.2014.925420. Epub 2014 Jun 10. Crit Rev Biochem Mol Biol. 2014. PMID: 24915502 Free PMC article. Review.
-
Biosynthesis of mycobacterial arabinogalactan: identification of a novel alpha(1-->3) arabinofuranosyltransferase.Mol Microbiol. 2008 Sep;69(5):1191-206. doi: 10.1111/j.1365-2958.2008.06354.x. Epub 2008 Jul 4. Mol Microbiol. 2008. PMID: 18627460 Free PMC article.
-
Controlled expression of branch-forming mannosyltransferase is critical for mycobacterial lipoarabinomannan biosynthesis.J Biol Chem. 2010 Apr 30;285(18):13326-36. doi: 10.1074/jbc.M109.077297. Epub 2010 Mar 9. J Biol Chem. 2010. PMID: 20215111 Free PMC article.
References
-
- Alderwick LJ, Radmacher E, Seidel M, Gande R, Hitchen PG, Morris HR, et al. Deletion of Cg-emb in corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J Biol Chem. 2005;280:32362–32371. - PubMed
-
- Alderwick LJ, Seidel M, Sahm H, Besra GS, Eggeling L. Identification of a Novel arabinofuranosyltransferase (AftA) involved in cell wall arabinan biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2006a;281:15653–15661. - PubMed
-
- Alderwick LJ, Dover LG, Seidel M, Gande R, Sahm H, Eggeling L, Besra GS. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-D-arabinose metabolism. Glycobiology. 2006b;16:1073–1081. - PubMed
-
- Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium–host interaction. Cell Microbiol. 2007;10:930–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

