Directional sensing during chemotaxis
- PMID: 18452713
- PMCID: PMC2519798
- DOI: 10.1016/j.febslet.2008.04.035
Directional sensing during chemotaxis
Abstract
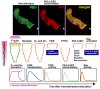
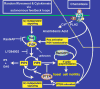
Cells have the innate ability to sense and move towards a variety of chemoattractants. We investigate the pathways by which cells sense and respond to chemoattractant gradients. We focus on the model system Dictyostelium and compare our understanding of chemotaxis in this system with recent advances made using neutrophils and other mammalian cell types, which share many molecular components and signaling pathways with Dictyostelium. This review also examines models that have been proposed to explain how cells are able to respond to small differences in ligand concentrations between the anterior leading edge and posterior of the cell. In addition, we highlight the overlapping functions of many signaling components in diverse processes beyond chemotaxis, including random cell motility and cell division.
Figures



References
-
- Rubel EW, Cramer KS. Choosing axonal real estate: location, location, location. J Comp Neurol. 2002;448:1–5. - PubMed
-
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. - PubMed
-
- Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. - PubMed
-
- Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–34. - PubMed
-
- Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

