Natural history of emphysema
- PMID: 18453357
- PMCID: PMC2645321
- DOI: 10.1513/pats.200802-018ET
Natural history of emphysema
Abstract
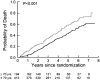
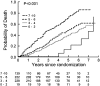
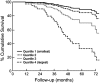
Chronic obstructive pulmonary disease (COPD) is a progressive disease with studies of disease progression generally focusing on measures of airflow and mortality. In nonsmokers, maximal lung function is attained around age 15 to 25 years, and after a variable plateau phase, subsequently declines at approximately 20 to 25 ml/year. Smoking may reduce the maximal FEV(1) achieved, shorten or eliminate the plateau phase, and may accelerate the rate of decline in lung function in a dose-dependent manner. Some smokers are predisposed to more rapid declines in lung function than others, and recent reports suggest that females may be at higher risk of lung damage related to smoke exposure than males. Progressive deterioration in dyspnea, functional status, and health-related quality of life (HRQL) in patients with COPD is well known, but the magnitude and rate of decline and its association with severity of airflow obstruction remains poorly defined. Many studies have identified pulmonary function, in particular the FEV(1), as the single best predictor of survival. An impaired diffusing capacity and overall impairment in functional status have also been associated with impaired survival in COPD. The National Emphysema Treatment Trial has provided additional insight into these features in a large, well-characterized group of patients with severe airflow obstruction and structural emphysema.
Figures
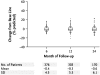
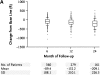

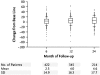
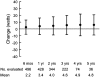
References
-
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. - PubMed
-
- World Health Organization. The GOLD global strategy for the management and prevention of COPD: Executive summary 2006. [Accessed 2007 Jul 21]. Available from: http://www.goldcopd.org.
-
- Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J 2003;22:268–273. - PubMed
-
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;152:S77–S121. - PubMed
Publication types
MeSH terms
Grants and funding
- N01HR76113/HR/NHLBI NIH HHS/United States
- N01HR76107/HR/NHLBI NIH HHS/United States
- N01HR76102/HR/NHLBI NIH HHS/United States
- N01HR76104/HR/NHLBI NIH HHS/United States
- N01HR76118/HR/NHLBI NIH HHS/United States
- N01HR76115/HR/NHLBI NIH HHS/United States
- N01HR76105/HR/NHLBI NIH HHS/United States
- N01HR76111/HR/NHLBI NIH HHS/United States
- N01HR76114/HR/NHLBI NIH HHS/United States
- N01HR76103/HR/NHLBI NIH HHS/United States
- N01HR76110/HR/NHLBI NIH HHS/United States
- N01HR76119/HR/NHLBI NIH HHS/United States
- N01HR76108/HR/NHLBI NIH HHS/United States
- N01HR76106/HR/NHLBI NIH HHS/United States
- N01HR76109/HR/NHLBI NIH HHS/United States
- N01HR76116/HR/NHLBI NIH HHS/United States
- N01HR76112/HR/NHLBI NIH HHS/United States
- N01HR76101/HR/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
