Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis
- PMID: 18453561
- PMCID: PMC2846244
- DOI: 10.4049/jimmunol.180.10.6457
Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis
Abstract
Plasmacytoid dendritic cells (pDCs) have both stimulatory and regulatory effects on T cells. pDCs are a major CNS-infiltrating dendritic cell population during experimental autoimmune encephalomyelitis but, unlike myeloid dendritic cells, have a minor role in T cell activation and epitope spreading. We show that depletion of pDCs during either the acute or relapse phases of experimental autoimmune encephalomyelitis resulted in exacerbation of disease severity. pDC depletion significantly enhanced CNS but not peripheral CD4(+) T cell activation, as well as IL-17 and IFN-gamma production. Moreover, CNS pDCs suppressed CNS myeloid dendritic cell-driven production of IL-17, IFN-gamma, and IL-10 in an IDO-independent manner. The data demonstrate that pDCs play a critical regulatory role in negatively regulating pathogenic CNS CD4(+) T cell responses, highlighting a new role for pDCs in inflammatory autoimmune disease.
Figures

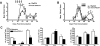

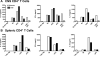
References
-
- Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. - PubMed
-
- Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. - PubMed
-
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

