Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques
- PMID: 18453600
- PMCID: PMC2596686
- DOI: 10.4049/jimmunol.180.10.6798
Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques
Abstract
Primate lentiviruses are typically apathogenic in their evolutionarily coadapted host species but can be lethal when transferred to new host species. Why such infections are pathogenic in humans and rhesus macaques (RMs) but not in sooty mangabeys (SMs), a natural host, remains unclear. Studies of chronically infected animals point to the importance of diminished immune activation in response to the infection in SMs. In this study, we sought the causes and timing of the differences in immune activation in a comparative study of acute SIV infection in RMs and SMs. Surprisingly, we show that in acute infection immune activation is comparable in SMs and RMs but thereafter, SMs quickly resolve immune activation, whereas RMs did not. Early resolution of immune activation in SMs correlated with increased expression of PD-1 and with preservation of CD4(+) T cell counts and lymphatic tissue architecture. These findings point to early control of immune activation by host immunoregulatory mechanisms as a major determinant of the different disease outcomes in SIV infection of natural vs non-natural hosts.
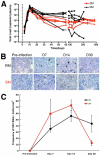
Figures







References
-
- Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J. Med. Primatol. 2005;34:243–252. - PubMed
-
- Johnson PR, Hirsch VM. Pathogenesis of AIDS: the non-human primate model. AIDS. 1991;5(Suppl 2):S43–S48. - PubMed
-
- Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI056997/AI/NIAID NIH HHS/United States
- AI 056997/AI/NIAID NIH HHS/United States
- N01 CO 12400/CO/NCI NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- AI 52755/AI/NIAID NIH HHS/United States
- R01 HL075766/HL/NHLBI NIH HHS/United States
- R01 AI 66998/AI/NIAID NIH HHS/United States
- R01 AI048484/AI/NIAID NIH HHS/United States
- RR 0165/RR/NCRR NIH HHS/United States
- R01 AI052755/AI/NIAID NIH HHS/United States
- N01 CO012400/CA/NCI NIH HHS/United States
- T32 AI 07421/AI/NIAID NIH HHS/United States
- R01 HL 75766/HL/NHLBI NIH HHS/United States
- T32 AI007421/AI/NIAID NIH HHS/United States
- R01 AI066998/AI/NIAID NIH HHS/United States
- R01 AI 48484/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

