Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses
- PMID: 18453607
- PMCID: PMC2408856
- DOI: 10.4049/jimmunol.180.10.6868
Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses
Abstract
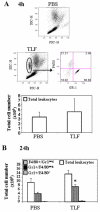
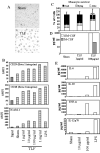
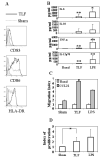
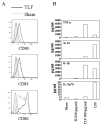
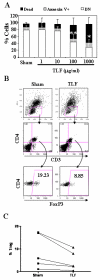
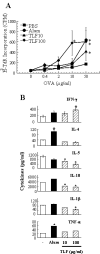
Lactoferrin is an 80-kDa iron-binding protein present at high concentrations in milk and in the granules of neutrophils. It possesses multiple activities, including antibacterial, antiviral, antifungal, and even antitumor effects. Most of its antimicrobial effects are due to direct interaction with pathogens, but a few reports show that it has direct interactions with cells of the immune system. In this study, we show the ability of recombinant human lactoferrin (talactoferrin alfa (TLF)) to chemoattract monocytes. What is more, addition of TLF to human peripheral blood or monocyte-derived dendritic cell cultures resulted in cell maturation, as evidenced by up-regulated expression of CD80, CD83, and CD86, production of proinflammatory cytokines, and increased capacity to stimulate the proliferation of allogeneic lymphocytes. When injected into the mouse peritoneal cavity, lactoferrin also caused a marked recruitment of neutrophils and macrophages. Immunization of mice with OVA in the presence of TLF promoted Th1-polarized Ag-specific immune responses. These results suggest that lactoferrin contributes to the activation of both the innate and adaptive immune responses by promoting the recruitment of leukocytes and activation of dendritic cells.
Figures

Similar articles
-
Alarmins link neutrophils and dendritic cells.Trends Immunol. 2009 Nov;30(11):531-7. doi: 10.1016/j.it.2009.07.004. Epub 2009 Aug 21. Trends Immunol. 2009. PMID: 19699678 Free PMC article. Review.
-
Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells.FASEB J. 2008 Aug;22(8):2747-57. doi: 10.1096/fj.07-098038. Epub 2008 Mar 25. FASEB J. 2008. PMID: 18364398
-
Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN+ (CD209) intermediate cell and impair differentiation into dendritic cells.J Immunol. 2003 Dec 15;171(12):6421-30. doi: 10.4049/jimmunol.171.12.6421. J Immunol. 2003. PMID: 14662841
-
Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics.J Exp Med. 1998 Apr 6;187(7):1019-28. doi: 10.1084/jem.187.7.1019. J Exp Med. 1998. PMID: 9529318 Free PMC article.
-
Immunomodulatory effects of lactoferrin on antigen presenting cells.Biochimie. 2009 Jan;91(1):11-8. doi: 10.1016/j.biochi.2008.05.005. Epub 2008 May 21. Biochimie. 2009. PMID: 18539153 Review.
Cited by
-
Immunological aspects of chronic venous disease pathogenesis.Cent Eur J Immunol. 2014;39(4):525-31. doi: 10.5114/ceji.2014.47740. Epub 2014 Dec 15. Cent Eur J Immunol. 2014. PMID: 26155174 Free PMC article. Review.
-
Acanthamoeba castellanii Genotype T4: Inhibition of Proteases Activity and Cytopathic Effect by Bovine Apo-Lactoferrin.Microorganisms. 2023 Mar 9;11(3):708. doi: 10.3390/microorganisms11030708. Microorganisms. 2023. PMID: 36985284 Free PMC article.
-
Lactoferrin in a Context of Inflammation-Induced Pathology.Front Immunol. 2017 Nov 6;8:1438. doi: 10.3389/fimmu.2017.01438. eCollection 2017. Front Immunol. 2017. PMID: 29163511 Free PMC article. Review.
-
Alarmins link neutrophils and dendritic cells.Trends Immunol. 2009 Nov;30(11):531-7. doi: 10.1016/j.it.2009.07.004. Epub 2009 Aug 21. Trends Immunol. 2009. PMID: 19699678 Free PMC article. Review.
-
Alarmins and Antitumor Immunity.Clin Ther. 2016 May;38(5):1042-53. doi: 10.1016/j.clinthera.2016.03.021. Epub 2016 Apr 22. Clin Ther. 2016. PMID: 27101817 Free PMC article. Review.
References
-
- Johanson B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960:510–512.
-
- Montreuil J, Tonnelat J, Mullet S. [Preparation and properties of lactosiderophilin (lactotransferrin) of human milk.] Biochim Biophys Acta. 1960;45:413–421. - PubMed
-
- Houghton MR, Gracey M, Burke V, Bottrell C, Spargo RM. Breast milk lactoferrin levels in relation to maternal nutritional status. J Pediatr Gastroenterol Nutr. 1985;4:230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

