Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways
- PMID: 18453609
- PMCID: PMC2637793
- DOI: 10.4049/jimmunol.180.10.6885
Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways
Abstract
The macrophage proinflammatory response to Francisella tularensis (Ft) live vaccine strain (LVS) was shown previously to be TLR2 dependent. The observation that intracellular Ft LVS colocalizes with TLR2 and MyD88 inside macrophages suggested that Ft LVS might signal from within the phagosome. Macrophages infected with LVSDeltaiglC, a Ft LVS mutant that fails to escape from the phagosome, displayed greatly increased expression of a subset of TLR2-dependent, proinflammatory genes (e.g., Tnf) but decreased expression of others (e.g., Ifnb1). This latter subset was similarly mitigated in IFN-beta(-/-) macrophages indicating that while Ft LVS-induced TLR2 signaling is necessary, cytosolic sensing of Ft to induce IFN-beta is required for full induction of the macrophage proinflammatory response. Although LVSDeltaiglC greatly increased IL-1beta mRNA in wild-type macrophages, protein secretion was not observed. IL-1beta secretion was also diminished in Ft LVS-infected IFN-beta(-/-) macrophages. rIFN-beta failed to restore IL-1beta secretion in LVSDeltaiglC-infected macrophages, suggesting that signals in addition to IFN-beta are required for assembly of the inflammasome and activation of caspase-1. IFN-beta plays a central role in controlling the macrophage bacterial burden: bacterial recovery was greater in IFN-beta(-/-) than in wild-type macrophages and treatment of Ft LVS-infected macrophages with rIFN-beta or 5,6-dimethylxanthenone-4-acetic acid, a potent IFN-beta inducer, greatly decreased the intracellular Ft LVS burden. In toto, these observations support the hypothesis that the host inflammatory response to Ft LVS is complex and requires engagement of multiple signaling pathways downstream of TLR2 including production of IFN-beta via an unknown cytosolic sensor and activation of the inflammasome.
Figures

 ) or LVSΔiglC (
) or LVSΔiglC (
 ) (MOI = 5) for 0–24 h. At the indicated time points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. Gene expression is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was enhanced (A) or diminished (B) by retention of the bacteria within the phagosome. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 3).
) (MOI = 5) for 0–24 h. At the indicated time points, total RNA was extracted from the macrophage cultures and analyzed by real-time PCR. Gene expression is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was enhanced (A) or diminished (B) by retention of the bacteria within the phagosome. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 3).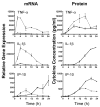
 ) or LVSΔiglC (
) or LVSΔiglC (
 ) (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α, IL-1β, and IP-10. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
) (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α, IL-1β, and IP-10. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).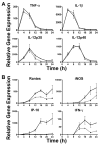
 ) or IFN-β−/− (
) or IFN-β−/− (
 ) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was unaffected (A) or diminished (B) by the lack of IFN-β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to macrophages exposed medium only. Displayed are genes whose expression was unaffected (A) or diminished (B) by the lack of IFN-β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).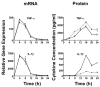
 ) or IFN-β−/− (
) or IFN-β−/− (
 ) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to peritoneal macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α and IL-1β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).
) mice were exposed to Ft LVS (MOI = 5) for 0–24 h. At the indicated time points, supernatants were collected and total RNA extracted from the macrophage cultures. Gene expression, analyzed by real-time PCR, is reported as relative gene expression compared to peritoneal macrophages exposed medium only. Supernatants were analyzed by ELISA for the presence of TNF-α and IL-1β. Data is presented as mean ± S.E.M. Data is derived from a single representative experiment (n = 2).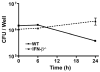
 ) or IFN-β−/− (
) or IFN-β−/− (
 ) mice were exposed to Ft LVS (MOI = 20) for 2 h. After a subsequent 1 h incubation in medium containing 50 μg/ml gentamicin, infected cells were washed 2× with PBS and then incubated with media alone. Cells were lysed and the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. (n=2).
) mice were exposed to Ft LVS (MOI = 20) for 2 h. After a subsequent 1 h incubation in medium containing 50 μg/ml gentamicin, infected cells were washed 2× with PBS and then incubated with media alone. Cells were lysed and the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. (n=2).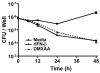
 ) or media supplemented with DMXAA (100 μg/ml) (
) or media supplemented with DMXAA (100 μg/ml) (
 ), rIFN-β (100 U/ml) (
), rIFN-β (100 U/ml) (
 ). Cells were lysed at the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 4)
). Cells were lysed at the indicated times and lysates were serially diluted and plated on MHA plates. Data is presented as mean ± S.E.M. Data is of a single representative experiment (n = 4)References
-
- Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. - PubMed
-
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. - PubMed
-
- Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II Respiratory challenge. Arch Intern Med. 1961;107:702–714. - PubMed
-
- Harris S. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann N Y Acad Sci. 1992;666:21–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

