Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun
- PMID: 18453624
- PMCID: PMC2872557
- DOI: 10.4049/jimmunol.180.10.7019
Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun
Abstract
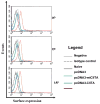
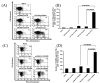
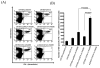
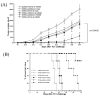
Administration of DNA vaccines via gene gun has emerged as an important form of Ag-specific immunotherapy. The MHC CIITA is a master regulator of MHC class II expression and also induces expression of class I molecules. We reasoned that the gene gun administration of CIITA DNA with DNA vaccines employing different strategies to improve MHC I and II processing could enhance DNA vaccine potency. We observed that DC-1 cells transfected with CIITA DNA lead to higher expression of MHC I and II molecules, leading to enhanced Ag presentation through the MHC I/II pathways. Furthermore, our data suggested that coadministration of DNA-encoding calreticulin (CRT) linked to human papillomavirus (HPV) 16 E6 Ag (CRT/E6) with CIITA DNA leads to enhanced E6-specific CD8(+) T cell immune responses in vaccinated mice. In addition, coadministration of the combination of CRT/E6 DNA with CIITA DNA and DNA encoding the invariant chain (Ii) linked to the pan HLA-DR-reactive epitope (Ii-PADRE) further enhanced E6-specific CD8(+) T cell immune responses in vaccinated mice. Treatment with the combination vaccine was also shown to enhance the antitumor effects and to prolong survival in TC-1 tumor-bearing mice. Vaccination with the combination vaccine also led to enhanced E6-specific CD8(+) memory T cells and to long-term protection against TC-1 tumors and prolonged survival in vaccinated mice. Thus, our findings suggest that the combination of CIITA DNA with CRT/E6 and Ii-PADRE DNA vaccines represents a potentially effective means to combat tumors in the clinical setting.
Figures



Similar articles
-
Innovative DNA vaccine for human papillomavirus (HPV)-associated head and neck cancer.Gene Ther. 2011 Mar;18(3):304-12. doi: 10.1038/gt.2010.151. Epub 2010 Oct 28. Gene Ther. 2011. PMID: 20981112 Free PMC article.
-
Histone deacetylase inhibitor AR-42 enhances E7-specific CD8⁺ T cell-mediated antitumor immunity induced by therapeutic HPV DNA vaccination.J Mol Med (Berl). 2013 Oct;91(10):1221-31. doi: 10.1007/s00109-013-1054-9. Epub 2013 May 29. J Mol Med (Berl). 2013. PMID: 23715898 Free PMC article.
-
Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell.Hum Gene Ther. 2007 Nov;18(11):1129-39. doi: 10.1089/hum.2007.090. Hum Gene Ther. 2007. PMID: 17939748 Free PMC article.
-
Modifying professional antigen-presenting cells to enhance DNA vaccine potency.Methods Mol Med. 2006;127:199-220. doi: 10.1385/1-59745-168-1:199. Methods Mol Med. 2006. PMID: 16988456 Review.
-
CIITA-Driven MHC Class II Expressing Tumor Cells as Antigen Presenting Cell Performers: Toward the Construction of an Optimal Anti-tumor Vaccine.Front Immunol. 2019 Jul 30;10:1806. doi: 10.3389/fimmu.2019.01806. eCollection 2019. Front Immunol. 2019. PMID: 31417570 Free PMC article. Review.
Cited by
-
Advances in Gene Delivery Systems.Pharmaceut Med. 2011 Oct 1;25(5):293-306. doi: 10.2165/11594020-000000000-00000. Pharmaceut Med. 2011. PMID: 22200988 Free PMC article.
-
Cross-protection against drifted influenza viruses: options offered by adjuvanted and intradermal vaccines.Hum Vaccin Immunother. 2013 Mar;9(3):582-90. doi: 10.4161/hv.23239. Epub 2013 Jan 7. Hum Vaccin Immunother. 2013. PMID: 23295230 Free PMC article. Review.
-
Induction of robust cellular immunity against HPV6 and HPV11 in mice by DNA vaccine encoding for E6/E7 antigen.Hum Vaccin Immunother. 2012 Apr;8(4):470-8. doi: 10.4161/hv.19180. Epub 2012 Feb 16. Hum Vaccin Immunother. 2012. PMID: 22336879 Free PMC article.
-
Targeted treatments for cervical cancer: a review.Onco Targets Ther. 2012;5:315-28. doi: 10.2147/OTT.S25123. Epub 2012 Nov 2. Onco Targets Ther. 2012. PMID: 23144564 Free PMC article.
-
Development of potent class II transactivator gene delivery systems capable of inducing de novo MHC II expression in human cells, in vitro and ex vivo.Gene Ther. 2017 Jun;24(6):342-352. doi: 10.1038/gt.2017.25. Epub 2017 Apr 17. Gene Ther. 2017. PMID: 28414303
References
-
- Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. - PubMed
-
- Hung CF, Yang M, Wu TC. Modifying professional antigen-presenting cells to enhance DNA vaccine potency. Methods Mol Med. 2006;127:199–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

