Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane
- PMID: 18453625
- PMCID: PMC2585549
- DOI: 10.4049/jimmunol.180.10.7028
Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane
Abstract
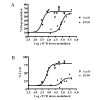
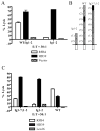
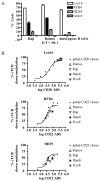
We have targeted CD22 as a novel tumor-associated Ag for recognition by human CTL genetically modified to express chimeric TCR (cTCR) recognizing this surface molecule. CD22-specific cTCR targeting different epitopes of the CD22 molecule promoted efficient lysis of target cells expressing high levels of CD22 with a maximum lytic potential that appeared to decrease as the distance of the target epitope from the target cell membrane increased. Targeting membrane-distal CD22 epitopes with cTCR(+) CTL revealed defects in both degranulation and lytic granule targeting. CD22-specific cTCR(+) CTL exhibited lower levels of maximum lysis and lower Ag sensitivity than CTL targeting CD20, which has a shorter extracellular domain than CD22. This diminished sensitivity was not a result of reduced avidity of Ag engagement, but instead reflected weaker signaling per triggered cTCR molecule when targeting membrane-distal epitopes of CD22. Both of these parameters were restored by targeting a ligand expressing the same epitope, but constructed as a truncated CD22 molecule to approximate the length of a TCR:peptide-MHC complex. The reduced sensitivity of CD22-specific cTCR(+) CTL for Ag-induced triggering of effector functions has potential therapeutic applications, because such cells selectively lysed B cell lymphoma lines expressing high levels of CD22, but demonstrated minimal activity against autologous normal B cells, which express lower levels of CD22. Thus, our results demonstrate that cTCR signal strength, and consequently Ag sensitivity, can be modulated by differential choice of target epitopes with respect to distance from the cell membrane, allowing discrimination between targets with disparate Ag density.
Figures




References
-
- Wang J, Press OW, Lindgren CG, Greenberg P, Riddell S, Qian X, Laugen C, Raubitschek A, Forman SJ, Jensen MC. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther. 2004;9:577–586. - PubMed
-
- Jensen MC, Clarke P, Tan G, Wright C, Chung-Chang W, Clark TN, Zhang F, Slovak ML, Wu AM, Forman SJ, Raubitschek A. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1:49–55. - PubMed
-
- Jensen M, Tan G, Forman S, Wu AM, Raubitschek A. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol Blood Marrow Transplant. 1998;4:75–83. - PubMed
-
- Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–1685. - PubMed
-
- Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

