KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos
- PMID: 18453744
- PMCID: PMC3632048
- DOI: 10.1159/000129628
KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos
Abstract
Several ion transporters have been implicated in left-right (LR) patterning. Here, we characterize a new component of the early bioelectrical circuit: the potassium channel KCNQ1 and its accessory subunit KCNE1. Having cloned the native Xenopus versions of both genes, we show that both are asymmetrically localized as maternal proteins during the first few cleavages of frog embryo development in a process dependent on microtubule and actin organization. Molecular loss-of-function using dominant negative constructs demonstrates that both gene products are required for normal LR asymmetry. We propose a model whereby these channels provide an exit path for K(+) ions brought in by the H(+),K(+)-ATPase. This physiological module thus allows the obligate but electroneutral H(+),K(+)-ATPase to generate an asymmetric voltage gradient on the left and right sides. Our data reveal a new, bioelectrical component of the mechanisms patterning a large-scale axis in vertebrate embryogenesis.
(c) 2008 S. Karger AG, Basel.
Figures
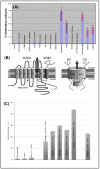
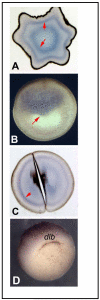
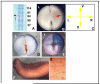
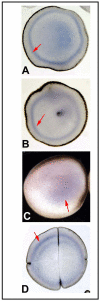
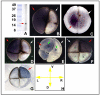
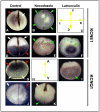
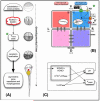
References
-
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: Current views and future potential. Physiol Rev. 2005;85:943–978. - PubMed
-
- Levin M. Large-scale biophysics: Ion flows and regeneration. Trends Cell Biol. 2007;17:262–271. - PubMed
-
- Cone CD. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30:151–181. - PubMed
-
- Levin M. Bioelectromagnetic patterning fields: Roles in embryonic development, regeneration, and neoplasm. Bioelectromagnetics. 2003;24:295–315.
-
- Robinson KR, Messerli MA. Left/right, up/down: The role of endogenous electrical fields as directional signals in development, repair and invasion. Bioessays. 2003;25:759–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
