Improving the photostability of bright monomeric orange and red fluorescent proteins
- PMID: 18454154
- PMCID: PMC2853173
- DOI: 10.1038/nmeth.1209
Improving the photostability of bright monomeric orange and red fluorescent proteins
Abstract
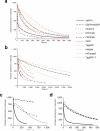
All organic fluorophores undergo irreversible photobleaching during prolonged illumination. Although fluorescent proteins typically bleach at a substantially slower rate than many small-molecule dyes, in many cases the lack of sufficient photostability remains an important limiting factor for experiments requiring large numbers of images of single cells. Screening methods focusing solely on brightness or wavelength are highly effective in optimizing both properties, but the absence of selective pressure for photostability in such screens leads to unpredictable photobleaching behavior in the resulting fluorescent proteins. Here we describe an assay for screening libraries of fluorescent proteins for enhanced photostability. With this assay, we developed highly photostable variants of mOrange (a wavelength-shifted monomeric derivative of DsRed from Discosoma sp.) and TagRFP (a monomeric derivative of eqFP578 from Entacmaea quadricolor) that maintain most of the beneficial qualities of the original proteins and perform as reliably as Aequorea victoria GFP derivatives in fusion constructs.
Figures

Comment in
-
Turning fluorescent proteins into energy-saving light bulbs.Nat Methods. 2008 Jun;5(6):472-3. doi: 10.1038/nmeth0608-472. Nat Methods. 2008. PMID: 18511915 No abstract available.
References
-
- Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. - PubMed
-
- Chudakov DM, et al. Photoswitchable cyan fluorescent protein for protein tracking. Nat Biotechnol. 2004;22:1435–1439. - PubMed
-
- Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

