Bortezomib/docetaxel combination therapy in patients with anthracycline-pretreated advanced/metastatic breast cancer: a phase I/II dose-escalation study
- PMID: 18454159
- PMCID: PMC2391111
- DOI: 10.1038/sj.bjc.6604347
Bortezomib/docetaxel combination therapy in patients with anthracycline-pretreated advanced/metastatic breast cancer: a phase I/II dose-escalation study
Abstract
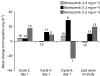
The aim of this study was to determine the dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) of bortezomib plus docetaxel in patients with anthracycline-pretreated advanced/metastatic breast cancer. Forty-eight patients received up to eight 21-day cycles of docetaxel (60-100 mg m(-2) on day 1) plus bortezomib (1.0-1.5 mg m(-2) on days 1, 4, 8, and 11). Pharmacodynamic and pharmacokinetic analyses were performed in a subset of patients. Five patients experienced DLTs: grade 3 bone pain (n=1) and febrile neutropenia (n=4). The MTD was bortezomib 1.5 mg m(-2) plus docetaxel 75 mg m(-2). All 48 patients were assessable for safety and efficacy. The most common adverse events were diarrhoea, nausea, alopecia, asthenia, and vomiting. The most common grade 3/4 toxicities were neutropenia (44%), and febrile neutropenia and diarrhoea (each 19%). Overall patient response rate was 29%. Median time to progression was 5.4 months. In patients with confirmed response, median time to response was 1.3 months and median duration of response was 3.2 months. At the MTD, response rate was 38%. Pharmacokinetic characteristics of bortezomib/docetaxel were comparable with single-agent data. Addition of docetaxel appeared not to affect bortezomib inhibition of 20S proteasome activity. Mean alpha-1 acid glycoprotein concentrations increased from baseline at nearly all time points across different bortezomib dose levels. Bortezomib plus docetaxel is an active combination for anthracycline-pretreated advanced/metastatic breast cancer. The safety profile is manageable and consistent with the side effects of the individual agents.
Figures
References
-
- Adams J (2002) Development of the proteasome inhibitor PS-341. Oncologist 7: 9–16 - PubMed
-
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ (1999) Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 59: 2615–2622 - PubMed
-
- Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, Canales C, Daud A, Spriggs DR (2002) A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 8: 2505–2511 - PubMed
-
- Aventis Pharmaceuticals Inc. (2005) Taxotere (Prescribing Information). Bridgewater, NJ: Aventis Pharmaceuticals Inc.
-
- Berns EM, Foekens JA, Vossen R, Look MP, Devilee P, Henzen-Logmans SC, van Staveren IL, van Putten WL, Inganas M, Meijer-van Gelder ME, Cornelisse C, Claassen CJ, Portengen H, Bakker B, Klijn JG (2000) Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res 60: 2155–2162 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

