DNA damage activates the SAC in an ATM/ATR-dependent manner, independently of the kinetochore
- PMID: 18454191
- PMCID: PMC2265443
- DOI: 10.1371/journal.pgen.1000015
DNA damage activates the SAC in an ATM/ATR-dependent manner, independently of the kinetochore
Abstract
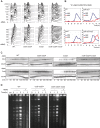
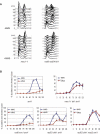
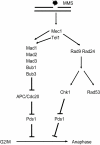
The DNA damage checkpoint and the spindle assembly checkpoint (SAC) are two important regulatory mechanisms that respond to different lesions. The DNA damage checkpoint detects DNA damage, initiates protein kinase cascades, and inhibits the cell cycle. The SAC relies on kinetochore-dependent assembly of protein complexes to inhibit mitosis when chromosomes are detached from the spindle. The two checkpoints are thought to function independently. Here we show that yeast cells lacking the DNA damage checkpoint arrest prior to anaphase in response to low doses of the DNA damaging agent methyl methane sulfonate (MMS). The arrest requires the SAC proteins Mad1, Mad2, Mad3, Bub1, and Bub3 and works through Cdc20 and Pds1 but unlike the normal SAC, does not require a functional kinetochore. Mec1 (ATR) and Tel1 (ATM) are also required, independently of Chk1 and Rad53, suggesting that Mec1 and Tel1 inhibit anaphase in response to DNA damage by utilizing SAC proteins. Our results demonstrate cross-talk between the two checkpoints and suggest that assembling inhibitory complexes of SAC proteins at unattached kinetochores is not obligatory for their inhibitory activity. Furthermore, our results suggest that there are novel, important targets of ATM and ATR for cell cycle regulation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. - PubMed
-
- Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. - PubMed
-
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. - PubMed
-
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

