Indolealkylamines: biotransformations and potential drug-drug interactions
- PMID: 18454322
- PMCID: PMC2751378
- DOI: 10.1208/s12248-008-9028-5
Indolealkylamines: biotransformations and potential drug-drug interactions
Abstract
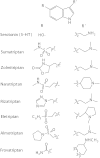
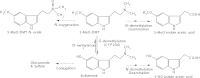
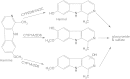
Indolealkylamine (IAA) drugs are 5-hydroxytryptamine (5-HT or serotonin) analogs that mainly act on the serotonin system. Some IAAs are clinically utilized for antimigraine therapy, whereas other substances are notable as drugs of abuse. In the clinical evaluation of antimigraine triptan drugs, studies on their biotransformations and pharmacokinetics would facilitate the understanding and prevention of unwanted drug-drug interactions (DDIs). A stable, principal metabolite of an IAA drug of abuse could serve as a useful biomarker in assessing intoxication of the IAA substance. Studies on the metabolism of IAA drugs of abuse including lysergic acid amides, tryptamine derivatives and beta-carbolines are therefore emerging. An important role for polymorphic cytochrome P450 2D6 (CYP2D6) in the metabolism of IAA drugs of abuse has been revealed by recent studies, suggesting that variations in IAA metabolism, pharmaco- or toxicokinetics and dynamics can arise from distinct CYP2D6 status, and CYP2D6 polymorphism may represent an additional risk factor in the use of these IAA drugs. Furthermore, DDIs with IAA agents could occur additively at the pharmaco/toxicokinetic and dynamic levels, leading to severe or even fatal serotonin toxicity. In this review, the metabolism and potential DDIs of these therapeutic and abused IAA drugs are described.
Figures






Similar articles
-
Clinically significant drug interactions with agents specific for migraine attacks.CNS Drugs. 2001;15(2):105-18. doi: 10.2165/00023210-200115020-00003. CNS Drugs. 2001. PMID: 11460889 Review.
-
Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions.Curr Drug Metab. 2010 Oct;11(8):659-66. doi: 10.2174/138920010794233495. Curr Drug Metab. 2010. PMID: 20942780 Free PMC article. Review.
-
Review: Pharmacogenetic aspects of the effect of cytochrome P450 polymorphisms on serotonergic drug metabolism, response, interactions, and adverse effects.Forensic Sci Med Pathol. 2011 Jun;7(2):162-84. doi: 10.1007/s12024-010-9188-3. Epub 2010 Nov 4. Forensic Sci Med Pathol. 2011. PMID: 21052868 Review.
-
Direct-acting antiviral interactions with opioids, alcohol or illicit drugs of abuse in HCV-infected patients.Liver Int. 2020 Jan;40(1):32-44. doi: 10.1111/liv.14283. Epub 2019 Nov 12. Liver Int. 2020. PMID: 31654604 Review.
-
Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials.Cephalalgia. 2002 Oct;22(8):633-58. doi: 10.1046/j.1468-2982.2002.00404.x. Cephalalgia. 2002. PMID: 12383060
Cited by
-
Pinoline may be used as a probe for CYP2D6 activity.Drug Metab Dispos. 2009 Mar;37(3):443-6. doi: 10.1124/dmd.108.025056. Epub 2008 Dec 18. Drug Metab Dispos. 2009. PMID: 19095720 Free PMC article.
-
The pharmacological profile and clinical prospects of the oral 5-HT1F receptor agonist lasmiditan in the acute treatment of migraine.Ther Adv Neurol Disord. 2015 Jan;8(1):46-54. doi: 10.1177/1756285614562419. Ther Adv Neurol Disord. 2015. PMID: 25584073 Free PMC article. Review.
-
Modification of 5-methoxy-N,N-dimethyltryptamine-induced hyperactivity by monoamine oxidase A inhibitor harmaline in mice and the underlying serotonergic mechanisms.Pharmacol Rep. 2016 Jun;68(3):608-15. doi: 10.1016/j.pharep.2016.01.008. Epub 2016 Feb 5. Pharmacol Rep. 2016. PMID: 26977821 Free PMC article.
-
Development of a mechanism-based pharmacokinetic/pharmacodynamic model to characterize the thermoregulatory effects of serotonergic drugs in mice.Acta Pharm Sin B. 2016 Sep;6(5):492-503. doi: 10.1016/j.apsb.2016.07.007. Epub 2016 Aug 6. Acta Pharm Sin B. 2016. PMID: 27709018 Free PMC article.
-
Potent inhibition of human organic cation transporter 2 (hOCT2) by β-carboline alkaloids.Xenobiotica. 2017 Dec;47(12):1112-1120. doi: 10.1080/00498254.2016.1271160. Epub 2017 Mar 2. Xenobiotica. 2017. PMID: 27977936 Free PMC article.
References
-
- Shulgin A. T., Shulgin A. TIKAL The Continuation. Berkeley, CA: Transform; 1997.
-
- Aghajanian G. K., Marek G. J. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21(2 Suppl):16S–23S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

