Cystathionine beta-synthase p.S466L mutation causes hyperhomocysteinemia in mice
- PMID: 18454451
- PMCID: PMC2630375
- DOI: 10.1002/humu.20773
Cystathionine beta-synthase p.S466L mutation causes hyperhomocysteinemia in mice
Abstract
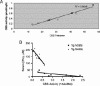
Missense mutations in the cystathionine beta-synthase (CBS) gene are the most common cause of clinical homocystinuria in humans. The p.S466L mutation was identified in a homocystinuric patient, but enzymatic studies with recombinant protein show this mutant to be highly active. To understand how this mutation causes disease in vivo, we have created mice lacking endogenous mouse CBS and expressing either wild-type (Tg-hCBS) or p.S466L (Tg-S466L) human CBS under control of zinc inducible metallothionein promoter. In the presence of zinc, we found that the mean serum total homocysteine (tHcy) of Tg-S466L mice was 142+/-55 microM compared to 16+/-13 microM for hCBS mice. Tg-S466L mice also had significantly higher levels of total free homocysteine and S-adenosylhomocysteine in liver and kidney. Only 48% of Tg-S466L mice had detectable CBS protein in the liver, whereas all the Tg-hCBS animals had detectable protein. Surprisingly, CBS mRNA was significantly elevated in Tg-S466L animals compared to Tg-hCBS, implying that the reduction in p.S466L protein was occurring due to posttranscriptional mechanisms. In Tg-S466L animals with detectable liver CBS, the enzyme formed tetramers and was active, but lacked inducibility by S-adenosylmethionine (AdoMet). However, even in Tg-S466L animals that had in vitro liver CBS activity equivalent to Tg-hCBS animals there was significant elevation of serum tHcy. Our results show that p.S466L causes homocystinuria by affecting both the steady state level of CBS protein and by reducing the efficiency of the enzyme in vivo.
Figures
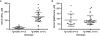



Similar articles
-
Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice.Circulation. 2009 Nov 10;120(19):1893-902. doi: 10.1161/CIRCULATIONAHA.109.866889. Epub 2009 Oct 26. Circulation. 2009. PMID: 19858416 Free PMC article.
-
Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia.FASEB J. 2009 Mar;23(3):883-93. doi: 10.1096/fj.08-120584. Epub 2008 Nov 5. FASEB J. 2009. PMID: 18987302 Free PMC article.
-
Analysis of the Qatari R336C cystathionine β-synthase protein in mice.J Inherit Metab Dis. 2019 Sep;42(5):831-838. doi: 10.1002/jimd.12140. Epub 2019 Jul 10. J Inherit Metab Dis. 2019. PMID: 31240737 Free PMC article.
-
The effect of dietary modulation of sulfur amino acids on cystathionine β synthase-deficient mice.Ann N Y Acad Sci. 2016 Jan;1363(1):80-90. doi: 10.1111/nyas.12967. Epub 2015 Nov 24. Ann N Y Acad Sci. 2016. PMID: 26599618 Free PMC article. Review.
-
The role of cystathionine beta-synthase in homocysteine metabolism.Antioxid Redox Signal. 2005 May-Jun;7(5-6):813-22. doi: 10.1089/ars.2005.7.813. Antioxid Redox Signal. 2005. PMID: 15890029 Review.
Cited by
-
Analysis of differential neonatal lethality in cystathionine β-synthase deficient mouse models using metabolic profiling.FASEB J. 2021 Jun;35(6):e21629. doi: 10.1096/fj.202100302R. FASEB J. 2021. PMID: 33949005 Free PMC article.
-
The c.797 G>A (p.R266K) cystathionine β-synthase mutation causes homocystinuria by affecting protein stability.Hum Mutat. 2017 Jul;38(7):863-869. doi: 10.1002/humu.23240. Epub 2017 May 22. Hum Mutat. 2017. PMID: 28488385 Free PMC article.
-
Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice.Blood. 2011 Aug 18;118(7):1998-2006. doi: 10.1182/blood-2011-01-333310. Epub 2011 Jun 8. Blood. 2011. PMID: 21653942 Free PMC article.
-
Cystathionine beta-synthase mutants exhibit changes in protein unfolding: conformational analysis of misfolded variants in crude cell extracts.J Inherit Metab Dis. 2012 May;35(3):469-77. doi: 10.1007/s10545-011-9407-4. Epub 2011 Nov 9. J Inherit Metab Dis. 2012. PMID: 22069143 Free PMC article.
-
Activation of mutant enzyme function in vivo by proteasome inhibitors and treatments that induce Hsp70.PLoS Genet. 2010 Jan;6(1):e1000807. doi: 10.1371/journal.pgen.1000807. Epub 2010 Jan 8. PLoS Genet. 2010. PMID: 20066033 Free PMC article.
References
-
- Gaustadnes M, Rudiger N, Rasmussen K, Ingerslev J. Intermediate and severe hyperhomocysteinemia with thrombosis: a study of genetic determinants. Thromb Haemost. 2000;83:554–558. - PubMed
-
- Guigo R. DNA composition, codon usage, and exon prediction. In: Bishop M, editor. Genetic databases. Cambridge, UK: Academic Press; 1999. pp. 53–79.
-
- Janosik M, Kery V, Gaustadnes M, Maclean KN, Kraus JP. Regulation of human cystathionine beta-synthase by S-adenosyl-L-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry. 2001;40:10625–10633. - PubMed
-
- Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7:813–822. - PubMed
-
- Kraus JP, Williamson CL, Firgaira FA, Yang-Feng TL, Munke M, Francke U, Rosenberg LE. Cloning and screening with nanogram amounts of immunopurified mRNAs: cDNA cloning and chromosomal mapping of cystathionine beta-synthase and the beta subunit of propionyl-CoA carboxylase. Proc Natl Acad Sci USA. 1986;83:2047–2051. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

