The dimeric structure of the cytochrome bc(1) complex prevents center P inhibition by reverse reactions at center N
- PMID: 18454936
- PMCID: PMC2495775
- DOI: 10.1016/j.bbabio.2008.04.008
The dimeric structure of the cytochrome bc(1) complex prevents center P inhibition by reverse reactions at center N
Abstract
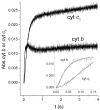
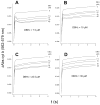
Energy transduction in the cytochrome bc(1) complex is achieved by catalyzing opposite oxido-reduction reactions at two different quinone binding sites. We have determined the pre-steady state kinetics of cytochrome b and c(1) reduction at varying quinol/quinone ratios in the isolated yeast bc(1) complex to investigate the mechanisms that minimize inhibition of quinol oxidation at center P by reduction of the b(H) heme through center N. The faster rate of initial cytochrome b reduction as well as its lower sensitivity to quinone concentrations with respect to cytochrome c(1) reduction indicated that the b(H) hemes equilibrated with the quinone pool through center N before significant catalysis at center P occurred. The extent of this initial cytochrome b reduction corresponded to a level of b(H) heme reduction of 33%-55% depending on the quinol/quinone ratio. The extent of initial cytochrome c(1) reduction remained constant as long as the fast electron equilibration through center N reduced no more than 50% of the b(H) hemes. Using kinetic modeling, the resilience of center P catalysis to inhibition caused by partial pre-reduction of the b(H) hemes was explained using kinetics in terms of the dimeric structure of the bc(1) complex which allows electrons to equilibrate between monomers.
Figures





References
-
- Zhang ZL, Huang LS, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. - PubMed
-
- Yu CA, Xia D, Kim H, Deisenhofer J, Zhang L, Kachurin AM, Yu L. Structural basis of functions of the mitochondrial bc1 complex. Biochim Biophys Acta. 1998;1365:151–158. - PubMed
-
- Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. - PubMed
-
- Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure at 2.3 Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–684. - PubMed
-
- Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-Ray Structure of Rhodobacter capsulatus cytochrome bc1: Comparison with its mitochondrial and chloroplast counterparts. Photosynth Res. 2004;81:251–275. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

