Survival of cancer cells is maintained by EGFR independent of its kinase activity
- PMID: 18455122
- PMCID: PMC2413063
- DOI: 10.1016/j.ccr.2008.03.015
Survival of cancer cells is maintained by EGFR independent of its kinase activity
Abstract
Expression of the epidermal growth factor receptor (EGFR), a receptor tyrosine kinase associated with cell proliferation and survival, is overactive in many tumors of epithelial origin. Blockade of the kinase activity of EGFR has been used for cancer therapy; however, by itself, it does not seem to reach maximum therapeutic efficacy. We report here that in human cancer cells, the function of kinase-independent EGFR is to prevent autophagic cell death by maintaining intracellular glucose level through interaction and stabilization of the sodium/glucose cotransporter 1 (SGLT1).
Figures
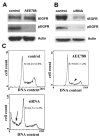


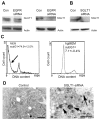
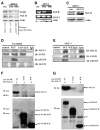

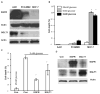
Comment in
-
A sweet new role for EGFR in cancer.Cancer Cell. 2008 May;13(5):375-6. doi: 10.1016/j.ccr.2008.04.008. Cancer Cell. 2008. PMID: 18455118
References
-
- Arteaga CL. Epidermal growth factor receptor dependence in human tumors: More than just expression? Oncologist. 2002;7 (Suppl 4):31–39. - PubMed
-
- Chan C, Gill GN. Mutational analysis of the nucleotide binding site of the epidermal growth factor receptor and v-Src protein-tyrosine kinases. J Biol Chem. 1996;271:22619–22623. - PubMed
-
- Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, Vokes EE. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–1987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

