Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression
- PMID: 18455429
- PMCID: PMC2953714
- DOI: 10.1016/j.semcancer.2008.03.011
Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression
Abstract
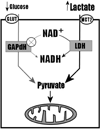
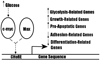
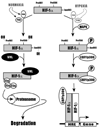
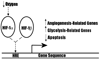
Carcinogenesis is a complex, multistep, multipath process often described as "somatic evolution". Conventional models of cancer progression are typically based on the genetic and epigenetic changes observed in malignant and premalignant tumors. We have explored an alternative approach that emphasizes the selection forces within adaptive landscapes governing growth and evolution in in situ, microinvasive, and metastatic cancers. In each environment, specific barriers to proliferation act as strong selection forces that determine the optimal phenotypic properties that permit tumor growth and invasion. Thus, the phenotypic properties or "hallmarks" of cancer can be viewed as successful adaptations to these microenvironmental selection forces. In turn, these selection pressures are not static but will dynamically change as a result of tumor population growth and evolution. Here, we emphasize the role of hypoxia and acidosis in the progression of tumor from in situ to invasive cancer. This is a consequence of early tumor cell proliferation on epithelial surfaces, which are separated from the underlying blood supply by the intact basement membrane. As tumor cells proliferate further away from the basement membrane, the diffusion-reaction kinetics of substrate and metabolite flow to and from the blood vessels result in regional hypoxia and acidosis. Cellular adaptation to the former include upregulation of glycolysis and to the latter include upregulation of Na+/H+ exchangers (NHE1) and other acid-regulating proteins such as carbonic anhydrase. We propose this phenotype is critical for subsequent malignant growth of primary and metastatic cancers.
Figures

References
-
- Foulds L. The experimental study of tumor progression: a review. Cancer Res. 1954;14:327–339. - PubMed
-
- Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J.Bioenerg.Biomembr. 2007;39:251–257. - PubMed
-
- Gatenby RA, Gillies RJ. A microenvironmental model of carcingenesis. Nature Rev. Cancer. 2008;8:56–61. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

