Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex
- PMID: 18455431
- PMCID: PMC2477738
- DOI: 10.1016/j.mcn.2008.03.003
Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex
Abstract
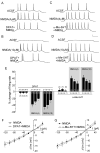
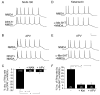
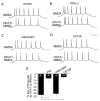
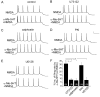
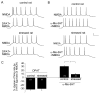
Both serotonin and NMDA signaling in prefrontal cortex (PFC) are implicated in mental disorders, including depression and anxiety. To understand their potential contributions to PFC neuronal excitability, we examined the effect of co-activation of 5-HT and NMDA receptors on action potential firing elicited by depolarizing current injection in PFC pyramidal neurons. In the presence of NMDA, a low concentration of the 5-HT(1A) agonist 8-OH-DPAT substantially reduced the number of spikes, and a low concentration of the 5-HT(2A/C) agonist alpha-Me-5HT significantly enhanced it, while both agonists were ineffective when applied alone. The 8-OH-DPAT effect on firing was mediated by inhibition of protein kinase A (PKA), whereas the alpha-Me-5HT effect was mediated by activation of protein kinase C (PKC). Moreover, the extracellular signal-regulated kinase (ERK), a signaling molecule downstream of PKA and PKC, was involved in both 5-HT(1A) and 5-HT(2A/C) modulation of neuronal excitability. Biochemical evidence showed that 5-HT(1A) decreased, whereas 5-HT(2A/C) increased the activation of ERK in an NMDA-dependent manner. In animals exposed to acute stress, the enhancing effect of 5-HT(2A/C) on firing was lost, while the decreasing effect of 5-HT(1A) on firing was intact. Concomitantly, the effect of 5-HT(2A/C), but not 5-HT(1A), on ERK activation was abolished in stressed animals. Taken together, our results demonstrate that distinct 5-HT receptor subtypes, by interacting with NMDA receptors, differentially regulate PFC neuronal firing, and the complex effects of 5-HT receptors on excitability are selectively altered under stressful conditions, which are often associated with mental disorders.
Figures
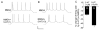
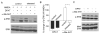
Similar articles
-
Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex.J Biol Chem. 2008 Jun 20;283(25):17194-204. doi: 10.1074/jbc.M801713200. Epub 2008 Apr 28. J Biol Chem. 2008. PMID: 18442977 Free PMC article.
-
A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex.Eur J Neurosci. 1999 Aug;11(8):2917-34. doi: 10.1046/j.1460-9568.1999.00708.x. Eur J Neurosci. 1999. PMID: 10457188
-
Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism.J Neurosci. 2005 Jun 8;25(23):5488-501. doi: 10.1523/JNEUROSCI.1187-05.2005. J Neurosci. 2005. PMID: 15944377 Free PMC article.
-
Single exposure to a serotonin 1A receptor agonist, (+)8-hydroxy-2-(di-n-propylamino)-tetralin, produces a prolonged heterologous desensitization of serotonin 2A receptors in neuroendocrine neurons in vivo.J Pharmacol Exp Ther. 2007 Mar;320(3):1078-86. doi: 10.1124/jpet.106.116004. Epub 2006 Dec 11. J Pharmacol Exp Ther. 2007. PMID: 17159160
-
Serotonergic regulation of neuronal excitability in the prefrontal cortex.Neuropharmacology. 2011 Sep;61(3):382-6. doi: 10.1016/j.neuropharm.2011.01.015. Epub 2011 Jan 18. Neuropharmacology. 2011. PMID: 21251917 Free PMC article. Review.
Cited by
-
5-HT1A receptor-regulated signal transduction pathways in brain.Cell Signal. 2010 Oct;22(10):1406-12. doi: 10.1016/j.cellsig.2010.03.019. Epub 2010 Apr 2. Cell Signal. 2010. PMID: 20363322 Free PMC article. Review.
-
Modulation of NMDA Receptors by G-protein-coupled receptors: Role in Synaptic Transmission, Plasticity and Beyond.Neuroscience. 2021 Feb 21;456:27-42. doi: 10.1016/j.neuroscience.2020.02.019. Epub 2020 Feb 24. Neuroscience. 2021. PMID: 32105741 Free PMC article. Review.
-
Restoration of glutamatergic transmission by dopamine D4 receptors in stressed animals.J Biol Chem. 2013 Sep 6;288(36):26112-26120. doi: 10.1074/jbc.M112.396648. Epub 2013 Jul 24. J Biol Chem. 2013. PMID: 23884421 Free PMC article.
-
Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence.Prog Neurobiol. 2018 Dec;171:32-49. doi: 10.1016/j.pneurobio.2018.10.001. Epub 2018 Oct 11. Prog Neurobiol. 2018. PMID: 30316901 Free PMC article. Review.
-
Selective serotonergic excitation of callosal projection neurons.Front Neural Circuits. 2012 Mar 20;6:12. doi: 10.3389/fncir.2012.00012. eCollection 2012. Front Neural Circuits. 2012. PMID: 22454619 Free PMC article.
References
-
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–41. - PubMed
-
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. - PubMed
-
- Aghajanian GK, Marek GJ. Serotonin–glutamate interactions: a new target for antipsychotic drugs. Neuropsychopharmacology. 1999;21:S122–S133.
-
- Andrade R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann N Y Acad Sci. 1998;861:190–203. - PubMed
-
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

