Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation
- PMID: 18455718
- PMCID: PMC2574794
- DOI: 10.1016/j.ydbio.2008.03.028
Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation
Abstract
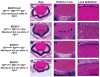
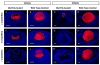
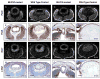
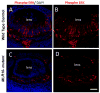
The vertebrate lens provides an excellent model to study the mechanisms that regulate terminal differentiation. Although fibroblast growth factors (FGFs) are thought to be important for lens cell differentiation, it is unclear which FGF receptors mediate these processes during different stages of lens development. Deletion of three FGF receptors (Fgfr1-3) early in lens development demonstrated that expression of only a single allele of Fgfr2 or Fgfr3 was sufficient for grossly normal lens development, while mice possessing only a single Fgfr1 allele developed cataracts and microphthalmia. Profound defects were observed in lenses lacking all three Fgfrs. These included lack of fiber cell elongation, abnormal proliferation in prospective lens fiber cells, reduced expression of the cell cycle inhibitors p27(kip1) and p57(kip2), increased apoptosis and aberrant or reduced expression of Prox1, Pax6, c-Maf, E-cadherin and alpha-, beta- and gamma-crystallins. Therefore, while signaling by FGF receptors is essential for lens fiber differentiation, different FGF receptors function redundantly.
Figures




Similar articles
-
Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation.Development. 2001 May;128(9):1617-27. doi: 10.1242/dev.128.9.1617. Development. 2001. PMID: 11290300
-
Role of cell and matrix-bound VEGF isoforms in lens development.Invest Ophthalmol Vis Sci. 2009 Jan;50(1):311-21. doi: 10.1167/iovs.08-2461. Epub 2008 Aug 29. Invest Ophthalmol Vis Sci. 2009. PMID: 18757513 Free PMC article.
-
Pax6 is essential for lens fiber cell differentiation.Development. 2009 Aug;136(15):2567-78. doi: 10.1242/dev.032888. Epub 2009 Jul 1. Development. 2009. PMID: 19570848 Free PMC article.
-
Exploring mechanisms of FGF signalling through the lens of structural biology.Nat Rev Mol Cell Biol. 2013 Mar;14(3):166-80. doi: 10.1038/nrm3528. Epub 2013 Feb 13. Nat Rev Mol Cell Biol. 2013. PMID: 23403721 Free PMC article. Review.
-
An essential role for FGF receptor signaling in lens development.Semin Cell Dev Biol. 2006 Dec;17(6):726-40. doi: 10.1016/j.semcdb.2006.10.002. Epub 2006 Oct 27. Semin Cell Dev Biol. 2006. PMID: 17116415 Free PMC article. Review.
Cited by
-
The aging mouse lens transcriptome.Exp Eye Res. 2021 Aug;209:108663. doi: 10.1016/j.exer.2021.108663. Epub 2021 Jun 11. Exp Eye Res. 2021. PMID: 34119483 Free PMC article.
-
Paralogous Genes Involved in Embryonic Development: Lessons from the Eye and Other Tissues.Genes (Basel). 2022 Nov 9;13(11):2082. doi: 10.3390/genes13112082. Genes (Basel). 2022. PMID: 36360318 Free PMC article. Review.
-
Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development.Development. 2010 Apr;137(7):1085-93. doi: 10.1242/dev.042820. Development. 2010. PMID: 20215346 Free PMC article.
-
The genomic basis of cichlid fish adaptation within the deepwater "twilight zone" of Lake Malawi.Evol Lett. 2017 Aug 29;1(4):184-198. doi: 10.1002/evl3.20. eCollection 2017 Sep. Evol Lett. 2017. PMID: 30283648 Free PMC article.
-
Activated Ras alters lens and corneal development through induction of distinct downstream targets.BMC Dev Biol. 2010 Jan 27;10:13. doi: 10.1186/1471-213X-10-13. BMC Dev Biol. 2010. PMID: 20105280 Free PMC article.
References
-
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–802. - PubMed
-
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–4393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

