Oxidoreductase regulation of Kv currents in rat ventricle
- PMID: 18455732
- PMCID: PMC2492761
- DOI: 10.1016/j.yjmcc.2008.03.011
Oxidoreductase regulation of Kv currents in rat ventricle
Abstract
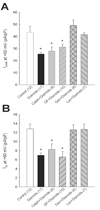
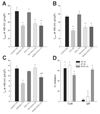
Oxidative stress contributes to the arrhythmogenic substrate created by myocardial ischemia-reperfusion partly through a shift in cell redox state, a key modulator of protein function. The activity of many oxidation-sensitive proteins is controlled by oxidoreductase systems that regulate the redox state of cysteine thiol groups, but the impact of these systems on ion channel function is not well defined. Thus, we examined the roles of the thioredoxin and glutaredoxin systems in controlling K(+) channels in the ventricle. An oxidative shift in redox state was elicited in isolated rat ventricular myocytes by brief exposure to diamide, a thiol-specific, membrane-permeable oxidant. Voltage-clamp studies showed that diamide decreased peak outward K(+) current (I(peak)) evoked by depolarizing test pulses by 41% (+60 mV; p<0.05) while steady-state outward current (I(ss)) measured at the end of the test pulse was decreased by 45% (p<0.05). These electrophysiological effects were not prevented by protein kinase C blockers, but the tyrosine kinase inhibitors genistein or lavendustin A blocked the suppression of both K(+) currents by diamide. Moreover, inhibition of I(peak) and I(ss) by diamide was reversed by dichloroacetate and an insulin-mimetic. The effect of dichloroacetate to normalize I(peak) after diamide was blocked by the thioredoxin system inhibitors auranofin or 13-cis-retinoic acid, but I(ss) was not affected by either compound. A pan-specific inhibitor of glutaredoxin and thioredoxin systems, 1,3-bis-(2-chloroethyl)-1-nitrosourea, also blocked the dichloroacetate effect on I(peak) but only partially inhibited the recovery of I(ss). These data suggest that acute regulation of cardiac K(+) channels by oxidoreductase systems is mediated by redox-sensitive tyrosine kinase/phosphatase pathways. The pathways controlling I(peak) channels are targets of the thioredoxin system whereas those regulating I(ss) channels are likely controlled by the glutaredoxin system. Thus, cardiac oxidoreductase systems may be important regulators of ion channels affected by pathogenic oxidative stress.
Figures




Similar articles
-
Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H416-24. doi: 10.1152/ajpheart.91446.2007. Epub 2008 May 30. Am J Physiol Heart Circ Physiol. 2008. PMID: 18515646 Free PMC article.
-
Redox control of K+ channel remodeling in rat ventricle.J Mol Cell Cardiol. 2006 Mar;40(3):339-49. doi: 10.1016/j.yjmcc.2005.09.019. Epub 2005 Nov 9. J Mol Cell Cardiol. 2006. PMID: 16288907
-
Redox regulation of Ito remodeling in diabetic rat heart.Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1417-24. doi: 10.1152/ajpheart.00559.2004. Epub 2004 Nov 11. Am J Physiol Heart Circ Physiol. 2005. PMID: 15539426
-
Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system.Am J Physiol Heart Circ Physiol. 2007 Mar;292(3):H1227-36. doi: 10.1152/ajpheart.01162.2006. Epub 2006 Dec 15. Am J Physiol Heart Circ Physiol. 2007. PMID: 17172268 Review.
-
Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems.Metallomics. 2018 Feb 21;10(2):218-228. doi: 10.1039/c7mt00327g. Metallomics. 2018. PMID: 29410996 Review.
Cited by
-
Role of gamma-glutamyl transpeptidase in redox regulation of K+ channel remodeling in postmyocardial infarction rat hearts.Am J Physiol Cell Physiol. 2009 Aug;297(2):C253-62. doi: 10.1152/ajpcell.00634.2008. Epub 2009 May 6. Am J Physiol Cell Physiol. 2009. PMID: 19419996 Free PMC article.
-
Redox control of cardiac excitability.Antioxid Redox Signal. 2013 Feb 1;18(4):432-68. doi: 10.1089/ars.2011.4234. Epub 2012 Aug 16. Antioxid Redox Signal. 2013. PMID: 22897788 Free PMC article. Review.
-
Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H416-24. doi: 10.1152/ajpheart.91446.2007. Epub 2008 May 30. Am J Physiol Heart Circ Physiol. 2008. PMID: 18515646 Free PMC article.
-
Redox Regulation of K+ Channel: Role of Thioredoxin.Antioxid Redox Signal. 2024 Nov;41(13-15):818-844. doi: 10.1089/ars.2023.0416. Epub 2024 Aug 28. Antioxid Redox Signal. 2024. PMID: 39099341 Free PMC article. Review.
-
Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5.Circ Res. 2012 Sep 14;111(7):842-53. doi: 10.1161/CIRCRESAHA.111.263525. Epub 2012 Jul 27. Circ Res. 2012. PMID: 22843785 Free PMC article.
References
-
- Powis G, Briehl M, Oblong L. Redox signaling and the control of cell growth and death. Pharm Ther. 1995;68(1):149–173. - PubMed
-
- Sen CK. Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol. 1998;55:1747–1758. - PubMed
-
- Paget MSB, Buttner MJ. Thiol-based regulatory switches. Ann Rev Genet. 2003;37:91–121. - PubMed
-
- Bigelow DJ, Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta. 2005;1703:121–134. - PubMed
-
- Tanaka T, Nakamura H, Nishiyama A, Hosoi F, Masutani H, Wada H, Yodoi J. Redox regulation by thioredoxin superfamily; Protection against oxidative stress and aging. Free Radical Res. 2000;33:851–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

